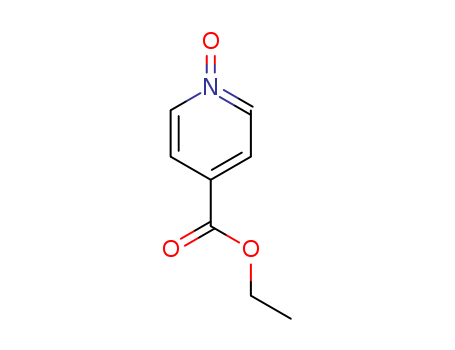
14906-37-7
- Product Name:N-Oxidized ethyl isonicotinate
- Molecular Formula:C8H9NO3
- Purity:99%
- Molecular Weight:167.164
Product Details
pd_meltingpoint:68-70 °C
Purity:99%
Chinese Factory Supply Wholesale N-Oxidized ethyl isonicotinate 14906-37-7 with Cheap Price
- Molecular Formula:C8H9NO3
- Molecular Weight:167.164
- Vapor Pressure:6.03E-05mmHg at 25°C
- Melting Point:68-70 °C
- Refractive Index:1.52
- Boiling Point:345.8 °C at 760 mmHg
- PKA:-0.37±0.10(Predicted)
- Flash Point:162.9 °C
- PSA:51.76000
- Density:1.16 g/cm3
- LogP:1.29180
Ethyl isonicotinate N-oxide(Cas 14906-37-7) Usage
|
General Description |
Ethyl isonicotinate N-oxide is a chemical compound generally utilized as an intermediate in the preparation of various chemical products. Structurally, it is an organic compound having an isonicotinic N-oxide moiety and an ethyl moiety attached to the nitrogen oxide atom. Details regarding its physical characteristics, general properties, health hazards, or environmental impact are not readily available, suggesting that limited scientific research has been conducted. Therefore, its safety, toxicity, and handling requirements aren't specifically known, therefore it should be handled with caution until more information is available. |
InChI:InChI=1/C8H9NO3/c1-2-12-8(10)7-3-5-9(11)6-4-7/h3-6H,2H2,1H3
14906-37-7 Relevant articles
4-Pyridyl carbonyl and related compounds as thrips lures: Effectiveness for onion thrips and New Zealand flower thrips in field experiments
Teulon, David A. J.,Davidson, Melanie M.,Hedderley, Duncan I.,James, Dale E.,Fletcher, Callum D.,Larsen, Lesley,Green, Vanessa C.,Perry, Nigel B.
, p. 6198 - 6205 (2007)
On the basis of structural and/or aroma ...
Evolutionary Design of Low Molecular Weight Organic Anolyte Materials for Applications in Nonaqueous Redox Flow Batteries
Sevov, Christo S.,Brooner, Rachel E. M.,Chénard, Etienne,Assary, Rajeev S.,Moore, Jeffrey S.,Rodríguez-López, Joaquín,Sanford, Melanie S.
, p. 14465 - 14472 (2015)
The integration of renewable energy sour...
Fluorovinylsulfones and -Sulfonates as Potent Covalent Reversible Inhibitors of the Trypanosomal Cysteine Protease Rhodesain: Structure-Activity Relationship, Inhibition Mechanism, Metabolism, and in Vivo Studies
Jung, Sascha,Fuchs, Natalie,Johe, Patrick,Wagner, Annika,Diehl, Erika,Yuliani, Tri,Zimmer, Collin,Barthels, Fabian,Zimmermann, Robert A.,Klein, Philipp,Waigel, Waldemar,Meyr, Jessica,Opatz, Till,Tenzer, Stefan,Distler, Ute,R?der, Hans-Joachim,Kersten, Christian,Engels, Bernd,Hellmich, Ute A.,Klein, Jochen,Schirmeister, Tanja
, p. 12322 - 12358 (2021/09/02)
Rhodesain is a major cysteine protease o...
From Pyridine- N-oxides to 2-Functionalized Pyridines through Pyridyl Phosphonium Salts: An Umpolung Strategy
Bugaenko, Dmitry I.,Yurovskaya, Marina A.,Karchava, Alexander V.
supporting information, p. 6099 - 6104 (2021/08/03)
The reactions of pyridine-N-oxides with ...
Reaction of Pyridine-N-Oxides with Tertiary sp2-N-Nucleophiles: An Efficient Synthesis of Precursors for N-(Pyrid-2-yl)-Substituted N-Heterocyclic Carbenes
Bugaenko, Dmitry I.,Karchava, Alexander V.,Yurovskaya, Marina A.
supporting information, p. 5777 - 5782 (2020/12/01)
N-(Pyrid-2-yl)-substituted azolium and p...
Visible Light-Mediated Decarboxylative Alkylation of Pharmaceutically Relevant Heterocycles
Sun, Alexandra C.,McClain, Edward J.,Beatty, Joel W.,Stephenson, Corey R. J.
supporting information, p. 3487 - 3490 (2018/06/26)
A net redox-neutral method for the decar...
14906-37-7 Process route
-
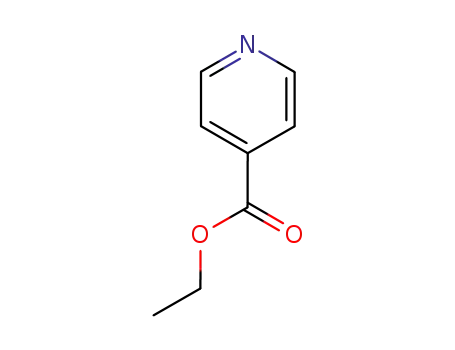
-
1570-45-2
isonicotinic acid ethylester

-
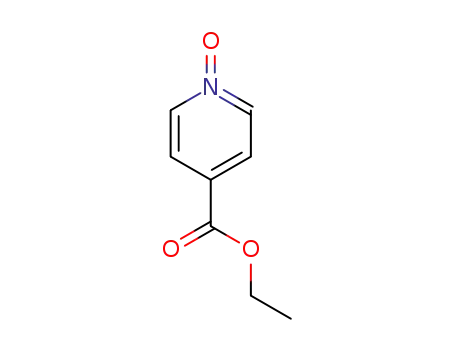
-
14906-37-7
ethyl isonicotinate N-oxide
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide;
In
acetic acid;
at 70 ℃;
Inert atmosphere;
|
100% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 20 ℃;
for 10h;
Sealed tube;
|
93% |
|
With
phthalic anhydride; urea-hydrogen peroxide;
In
dichloromethane;
for 4h;
Ambient temperature;
|
90% |
|
With
urea hydrogen peroxide adduct; trifluoroacetic anhydride;
In
acetonitrile;
at 0 ℃;
for 0.166667h;
|
83% |
|
|
80% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 ℃;
for 16h;
|
29% |
|
With
phthalic anhydride; dihydrogen peroxide;
|
|
|
With
dihydrogen peroxide; acetic acid;
|
|
|
With
dihydrogen peroxide; acetic acid;
at 75 ℃;
for 24h;
|
10.5 g |
|
With
dihydrogen peroxide; acetic acid;
In
water;
at 75 ℃;
for 24h;
|
|
|
isonicotinic acid ethylester;
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 24 ℃;
for 12h;
With
triphenylphosphine;
In
dichloromethane;
for 4h;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 24h;
|
|
|
isonicotinic acid ethylester;
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 24h;
With
potassium carbonate;
In
dichloromethane; water;
at 20 ℃;
for 0.5h;
|
-
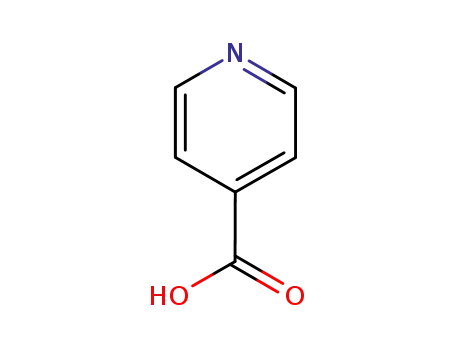
-
55-22-1
pyridine-4-carboxylic acid

-

-
14906-37-7
ethyl isonicotinate N-oxide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sulfuric acid / ethanol / 24 h / Reflux
2: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 16 h / 0 °C
With
sulfuric acid; 3-chloro-benzenecarboperoxoic acid;
In
ethanol; dichloromethane;
|
14906-37-7 Upstream products
-
1570-45-2

isonicotinic acid ethylester
-
55-22-1

pyridine-4-carboxylic acid
14906-37-7 Downstream products
-
6313-54-8
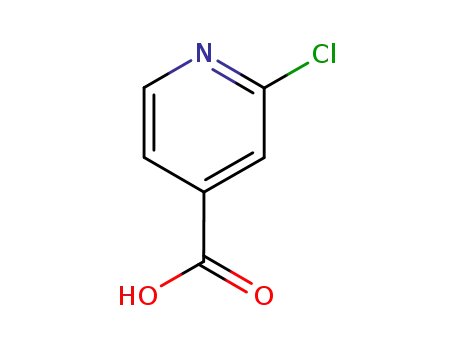
2-chloroisonicotinic acid,
-
38557-82-3
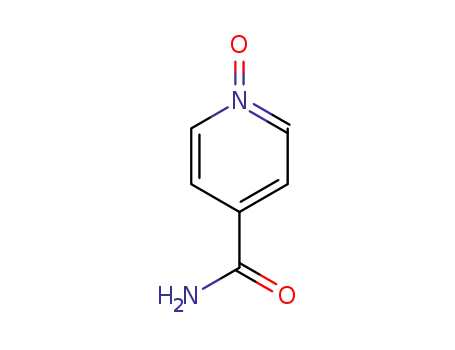
4-pyridine carboxamide 1-oxide
-
6975-73-1
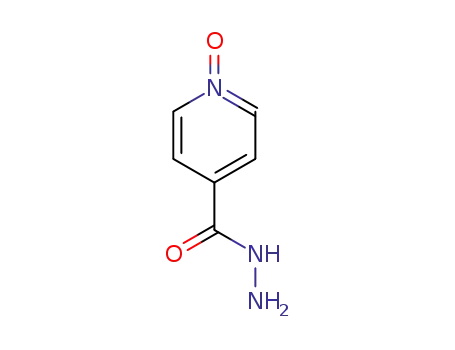
1-oxide pyridine-4-carbohydrazide
-
100868-12-0
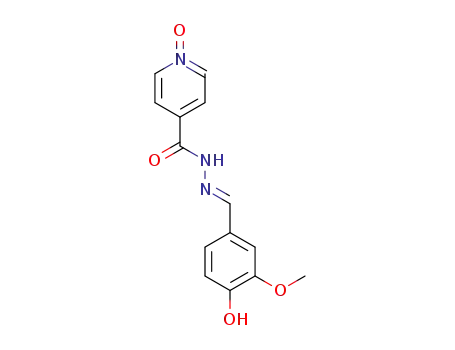
1-oxy-isonicotinic acid vanillylidenehydrazide
Relevant Products
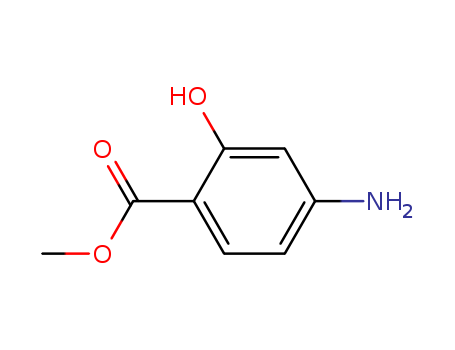
-
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
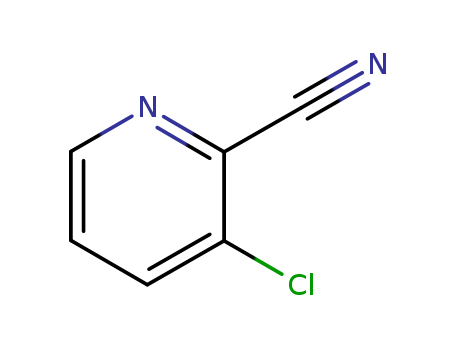
-
3-chloro-2-cyanopyridine
CAS:38180-46-0
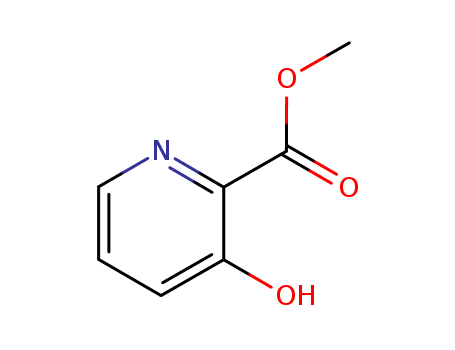
-
Methyl 3-hydroxy-2-pyridinecarboxylate
CAS:62733-99-7