62733-99-7
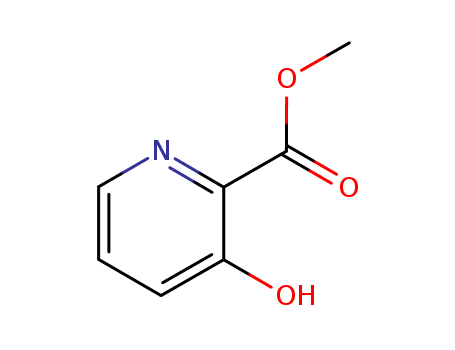
- Product Name:Methyl 3-hydroxy-2-pyridinecarboxylate
- Molecular Formula:C7H7NO3
- Purity:99%
- Molecular Weight:153.137
Product Details
pd_meltingpoint:75.0 to 79.0 °C
Purity:99%
Factory Sells Best Quality Methyl 3-hydroxy-2-pyridinecarboxylate 62733-99-7 with USP
- Molecular Formula:C7H7NO3
- Molecular Weight:153.137
- Vapor Pressure:4.3E-05mmHg at 25°C
- Melting Point:75.0 to 79.0 °C
- Refractive Index:1.551
- Boiling Point:340.6 °C at 760 mmHg
- PKA:4.45±0.10(Predicted)
- Flash Point:159.8 °C
- PSA:59.42000
- Density:1.287 g/cm3
- LogP:0.57380
3-HYDROXY-PYRIDINE-2-CARBOXYLIC ACID METHYL ESTER(Cas 62733-99-7) Usage
|
General Description |
3-Hydroxy-pyridine-2-carboxylic acid methyl ester, also known as 2-Hydroxynicotinic acid methyl ester, is a chemical compound with the molecular formula C7H7NO3. It is a derivative of nicotinic acid and is commonly used in the pharmaceutical industry as a building block for the synthesis of various pharmaceutical drugs and active ingredients. This chemical compound has been studied for its potential biological activities and medicinal properties, including its anti-inflammatory and antioxidant effects. It may also have potential applications in the field of agrochemicals and crop protection. Additionally, 3-Hydroxy-pyridine-2-carboxylic acid methyl ester has been investigated for its potential use in the development of new materials and biologically active compounds for diverse industrial applications. |
InChI:InChI=1/C7H7NO3/c1-11-7(10)6-5(9)3-2-4-8-6/h2-4,9H,1H3
62733-99-7 Relevant articles
2-(aza-9-fluorenonyl)carbapenem antibacterial agents
-
, (2008/06/13)
Carbapenems of the formula STR1 wherein ...
62733-99-7 Process route
-
-
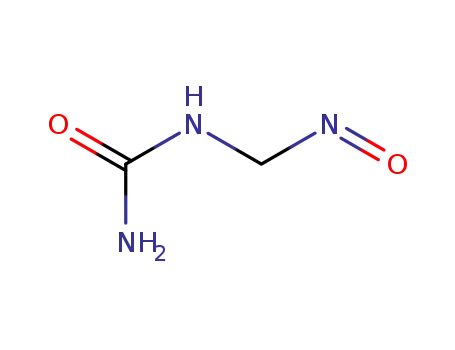
36851-80-6
nitrosomethylurea

-
-
874-24-8
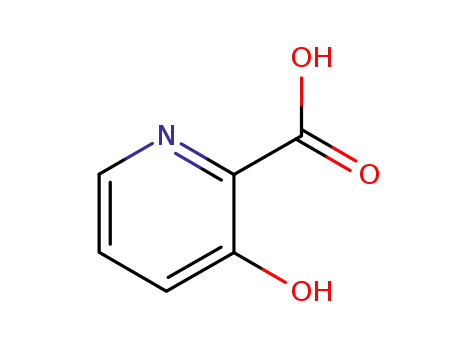
3-hydroxypyridine-2-carboxylic acid

-
-
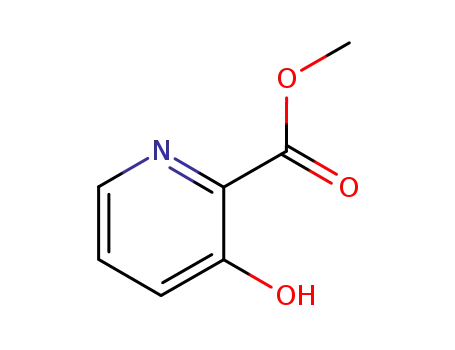
62733-99-7
methyl 3-hydroxypicolinate
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
In
tetrahydrofuran;
|
-

-
62733-99-7
methyl 3-hydroxypicolinate

-
-
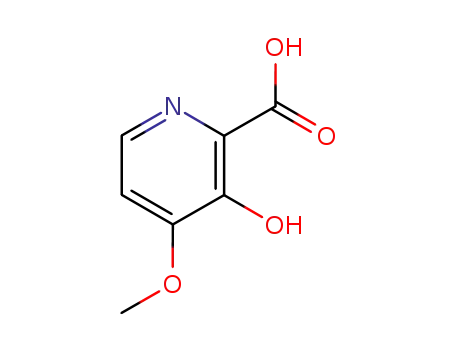
210300-09-7
3-hydroxy-4-methoxy-pyridine-2-carboxylic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: bromine / water / Inert atmosphere
2.1: sodium hydride / N,N-dimethyl-formamide / 0.25 h / 20 °C / Inert atmosphere
2.2: 6 h / 90 °C / Inert atmosphere
3.1: potassium carbonate / 30 h / Reflux; Inert atmosphere
4.1: triethylamine; hydrogen; 5%-palladium/activated carbon / ethanol / 3 h / 2327.23 - 2585.81 Torr
With
5%-palladium/activated carbon; hydrogen; bromine; sodium hydride; potassium carbonate; triethylamine;
In
ethanol; water; N,N-dimethyl-formamide;
|
62733-99-7 Upstream products
-
36851-80-6

nitrosomethylurea
-
874-24-8

3-hydroxypyridine-2-carboxylic acid
62733-99-7 Downstream products
-
122019-56-1
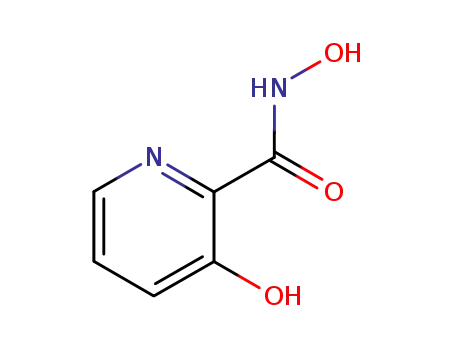
N,3-dihydroxy-2-pyridinecarboxamide
-
948836-33-7
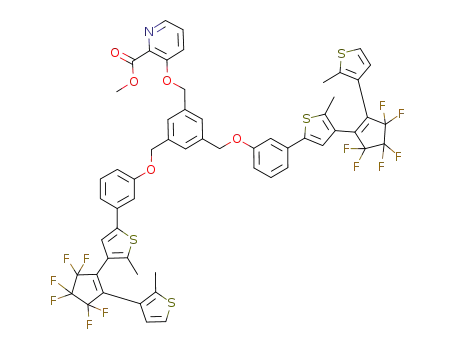
C58H41F12NO5S4
-
948836-30-4
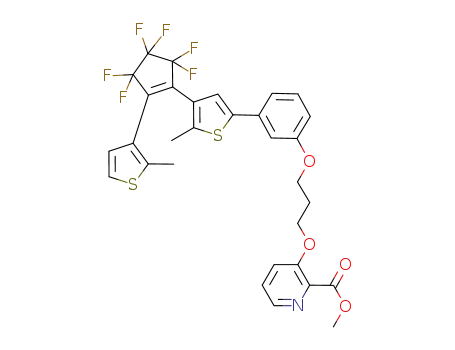
3-[3-(3-{4-[3,3,4,4,5,5-hexafluoro-2-(2-methylthiophen-3-yl)cyclopent-1-enyl]-5-methylthiophen-2-yl}phenoxy)propoxy]pyridine-2-carboxylic acid methyl ester
-
948836-31-5
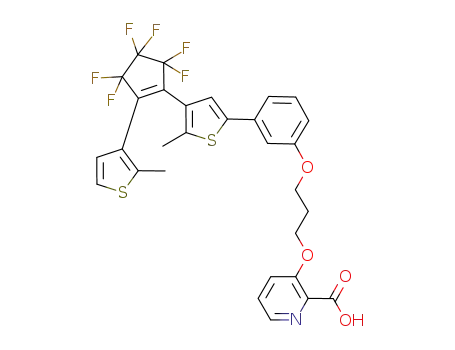
3-[3-(3-{4-[3,3,4,4,5,5-hexafluoro-2-(2-methylthiophen-3-yl)cyclopent-1-enyl]-5-methylthiophen-2-yl}phenoxy)propoxy]pyridine-2-carboxylic acid
Relevant Products
-
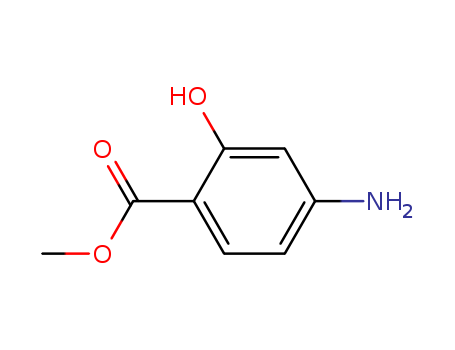
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
-
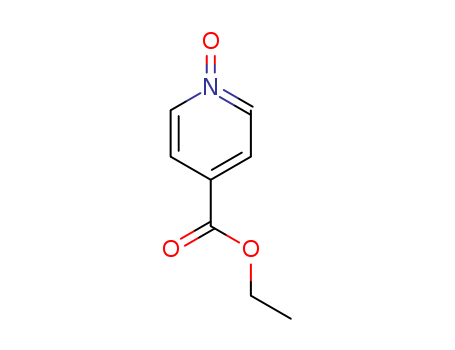
N-Oxidized ethyl isonicotinate
CAS:14906-37-7
-
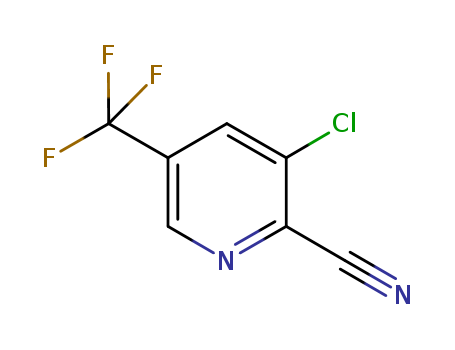
3-chloro-2-cyano-5-trifluoromethylpyridine
CAS:80194-70-3