328086-60-8
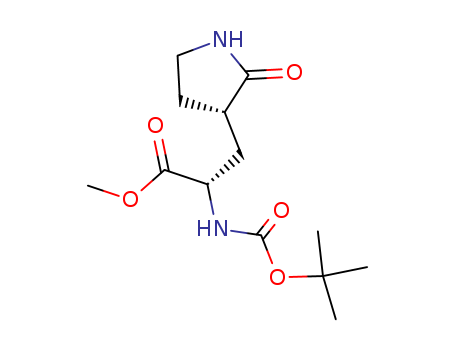
- Product Name:(S) 2- (Boc-amino) -3- [(S) -2-oxo-3-pyrrolidinyl] propionic acid methyl ester
- Molecular Formula:
- Purity:99%
- Molecular Weight:286.328
Product Details
pd_meltingpoint:110-114°C
Purity:99%
Factory supply good quality (S) 2- (Boc-amino) -3- [(S) -2-oxo-3-pyrrolidinyl] propionic acid methyl ester 328086-60-8 with stock
- Molecular Formula:C13H22N2O5
- Molecular Weight:286.328
- Melting Point:110-114°C
- Boiling Point:471.5±20.0 °C(Predicted)
- PKA:10.97±0.46(Predicted)
- Density:1.142±0.06 g/cm3(Predicted)
Company Profile
Bailong Pharmaceutical officially began operations in 2017, located in the High-tech Zone of Jinan City. It is a high-tech enterprise specializing in the research and production of chemical raw materials and advanced pharmaceutical intermediates, and has currently passed the GB/T 19001-2016/ISO 9001 quality management system certification standards. The company is dedicated to serving major pharmaceutical companies, fine chemical production units, large pharmaceutical research and development centers, and other users of pharmaceutical chemicals both domestically and internationally. It has established cooperation with nearly all of the top ten pharmaceutical companies in the country and hundreds of small and medium-sized biotechnology companies, providing specialized services such as the supply of commercial chemicals, technical research and development, production technology outsourcing, and customized chemicals. In 2020, it was included in the list of "Top Ten Suppliers of the Year" for certain A-share listed companies, reaching a long-term cooperation intention to help clients improve production capacity and development efficiency, and push the project to market at full speed.

Development History
- 2020yearThe application for Shandong high-end pharmaceutical intermediates production base was successful, and it was officially put into operation in June 2022
- 2021yearIn the first quarter, the growth rate reached a new high, with a year-on-year increase of 210%, achieving leapfrog growth in the new fiscal year
- 2022yearIn December,the independent R&D laboratory building of Yinfeng Bio-City was put into operation to help product R&D and innovation.
328086-60-8 Relevant articles
Improved Synthesis of a Cyclic Glutamine Analogue Used in Antiviral Agents Targeting 3C and 3CL Proteases including SARS-CoV-2 Mpro
Vuong, Wayne,Vederas, John C.
, p. 13104 - 13110 (2021)
An intermediate in the synthesis of nume...
Design, synthesis, and biological evaluation of peptidomimetic aldehydes as broad-spectrum inhibitors against enterovirus and sars-cov-2
Dai, Wenhao,Jochmans, Dirk,Xie, Hang,Yang, Hang,Li, Jian,Su, Haixia,Chang, Di,Wang, Jiang,Peng, Jingjing,Zhu, Lili,Nian, Yong,Hilgenfeld, Rolf,Jiang, Hualiang,Chen, Kaixian,Zhang, Leike,Xu, Yechun,Neyts, Johan,Liu, Hong
, p. 2794 - 2808 (2021/05/06)
A novel series of peptidomimetic aldehyd...
KETOAMIDE COMPOUND AND PREPARATION METHOD, PHARMACEUTICAL COMPOSITION, AND USE THEREOF
-
Paragraph 0098; 0100, (2021/06/21)
A ketoamide compound and a preparation m...
ANTIVIRAL COMPOUNDS FOR THE TREATMENT OF CORONAVIRUS, PICORNAVIRUS AND NOROVIRUS INFECTIONS
-
Paragraph 0165; 0167, (2021/12/31)
Provided herein are compounds of Formula...
A Quick Route to Multiple Highly Potent SARS-CoV-2 Main Protease Inhibitors**
Yang, Kai S.,Ma, Xinyu R.,Ma, Yuying,Alugubelli, Yugendar R.,Scott, Danielle A.,Vatansever, Erol C.,Drelich, Aleksandra K.,Sankaran, Banumathi,Geng, Zhi Z.,Blankenship, Lauren R.,Ward, Hannah E.,Sheng, Yan J.,Hsu, Jason C.,Kratch, Kaci C.,Zhao, Baoyu,Hayatshahi, Hamed S.,Liu, Jin,Li, Pingwei,Fierke, Carol A.,Tseng, Chien-Te K.,Xu, Shiqing,Liu, Wenshe Ray
supporting information, p. 942 - 948 (2020/12/15)
The COVID-19 pathogen, SARS-CoV-2, requi...
328086-60-8 Process route
-
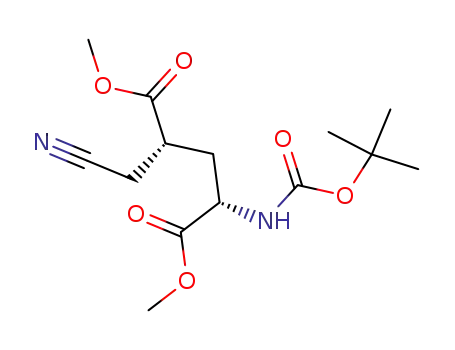
- 328086-57-3
(2S,4S)-2-tert-butoxycarbonylamino-4-cyanomethyl-pentadioic acid dimethyl ester

-
![(2S)-2-(tert-butoxycarbonylamino)-3-[(3'S)-2'-oxo-3'-pyrrolidinyl]propanoic acid methy ester](/upload/2025/3/250e4118-3ae7-4350-8ead-10757f0bfce4.png)
- 328086-60-8
(2S)-2-(tert-butoxycarbonylamino)-3-[(3'S)-2'-oxo-3'-pyrrolidinyl]propanoic acid methy ester
| Conditions | Yield |
|---|---|
|
With sodium tetrahydridoborate; CoCl2·6H2O; In methanol; at 20 ℃; for 12h; Cooling with ice;
|
55% |
|
With sodium tetrahydridoborate; CoCl2·6H2O; In methanol; at 20 ℃; for 12h;
|
52.6% |
|
1,5-dimethyl (2S,4R)-2-[(tert-butoxycarbonyl)amino]-4-(cyanomethyl)pentanedioate; With CoCl2·6H2O; In methanol; at 0 ℃; for 0.166667h;
With sodium tetrahydridoborate; In methanol; at 20 ℃; for 16h; Cooling with ice;
|
51% |
|
With sodium tetrahydridoborate; CoCl2·6H2O; In methanol; at 0 - 20 ℃; for 12h;
|
40% |
|
1,5-dimethyl (2S,4R)-2-[(tert-butoxycarbonyl)amino]-4-(cyanomethyl)pentanedioate; With Adams’s catalyst; hydrogen; In methanol; chloroform; at 20 ℃; for 36h;
With anhydrous sodium carbonate; In methanol; chloroform; for 6h; Reflux;
|
37% |
|
Multi-step reaction with 2 steps
1: H2 / Pd/C / 2 h / 3620.04 Torr
2: 5.55 g / Et3N / tetrahydrofuran / 60 °C
With hydrogen; triethylamine; palladium on activated charcoal; In tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1: H2 / PtO2 / methanol; CHCl3 / 12 h / 25 °C
2: NaOAc / methanol; CHCl3 / 12 h / Heating
With hydrogen; anhydrous Sodium acetate; Adams’s catalyst; In methanol; chloroform;
|
|
|
Multi-step reaction with 2 steps
1: H2 / PtO2 / CHCl3; methanol / 36 h / 2585.74 Torr
2: 4.2 g / Na2CO3 / CHCl3; methanol / 6 h / pH 7 / Heating
With hydrogen; anhydrous sodium carbonate; Adams’s catalyst; In methanol; chloroform;
|
|
|
Multi-step reaction with 2 steps
1: hydrogen / PtO2 / methanol; CHCl3 / 2585.74 Torr
2: Na2CO3 / methanol; CHCl3 / 60 °C
With hydrogen; anhydrous sodium carbonate; Adams’s catalyst; In methanol; chloroform;
|
|
|
With sodium tetrahydridoborate; cobalt(II) chloride; In methanol; at 0 - 20 ℃;
|
3.19 g |
|
Multi-step reaction with 2 steps
1: Adams’s catalyst; hydrogen / methanol; chloroform / 12 h / 20 °C
2: anhydrous Sodium acetate / methanol; chloroform / 12 h / 60 °C
With Adams’s catalyst; hydrogen; anhydrous Sodium acetate; In methanol; chloroform;
|
|
|
Multi-step reaction with 2 steps
1: platinum(IV) oxide; hydrogen / chloroform; methanol / 12 h / 20 °C
2: anhydrous Sodium acetate / methanol / 12 h / 60 °C
With platinum(IV) oxide; hydrogen; anhydrous Sodium acetate; In methanol; chloroform;
|
|
|
With methanol; sodium tetrahydridoborate; CoCl2·6H2O; at 0 - 20 ℃; for 24.5h;
|
2.1 g |
-
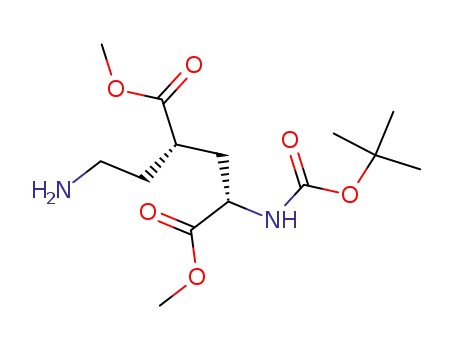
- 328086-58-4
(2S,4S)-2-(2-Amino-ethyl)-4-tert-butoxycarbonylamino-pentanedioic acid dimethyl ester

-
![(2S)-2-(tert-butoxycarbonylamino)-3-[(3'S)-2'-oxo-3'-pyrrolidinyl]propanoic acid methy ester](/upload/2025/3/250e4118-3ae7-4350-8ead-10757f0bfce4.png)
- 328086-60-8
(2S)-2-(tert-butoxycarbonylamino)-3-[(3'S)-2'-oxo-3'-pyrrolidinyl]propanoic acid methy ester
| Conditions | Yield |
|---|---|
|
With sodium carbonate; In methanol; chloroform; for 6h; pH=7; Heating;
|
4.2 g |
|
With sodium acetate; In methanol; chloroform; for 12h; Heating;
|
|
|
(2S,4S)-2-(2-Amino-ethyl)-4-tert-butoxycarbonylamino-pentanedioic acid dimethyl ester; With triethylamine; In tetrahydrofuran; at 60 ℃;
With water; In tetrahydrofuran;
|
|
|
With sodium acetate; In methanol; chloroform; at 60 ℃; for 12h;
|
|
|
With sodium acetate; In methanol; at 60 ℃; for 12h;
|
2.2 g |
328086-60-8 Upstream products
-
328086-58-4

(2S,4S)-2-(2-Amino-ethyl)-4-tert-butoxycarbonylamino-pentanedioic acid dimethyl ester
-
56-86-0
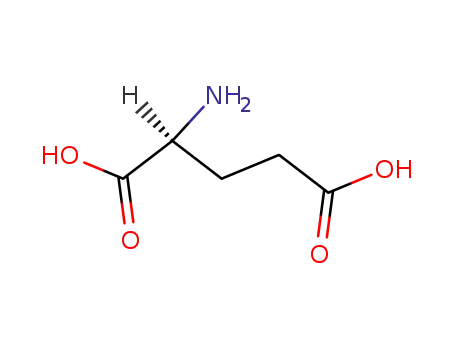
L-glutamic acid
-
6525-53-7
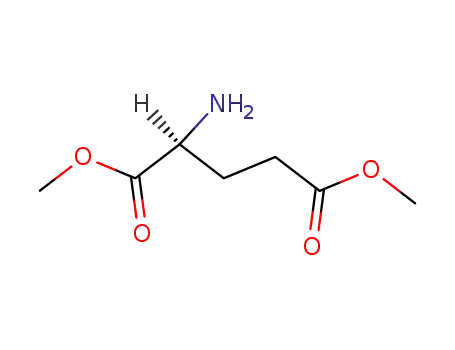
L-glutamic acid dimethyl ester
-
59279-60-6
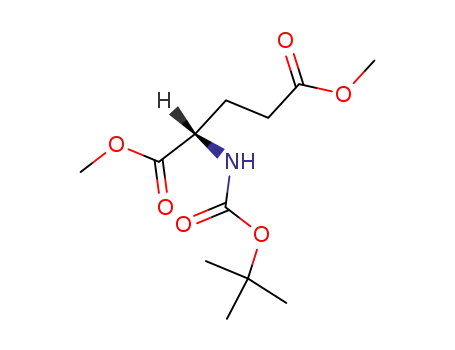
dimethyl (2S)-2-[(tert-butoxycarbonyl)amino]pentanedioate
328086-60-8 Downstream products
-
1027038-43-2
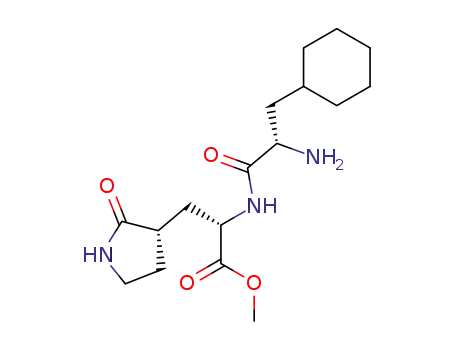
methyl (2S)-2-[[(2S)-2-amino-3-cyclohexyl-propanoyl]amino]-3-[(3S)-2-oxopyrrolidin-3-yl]propanoate
-
898264-60-3
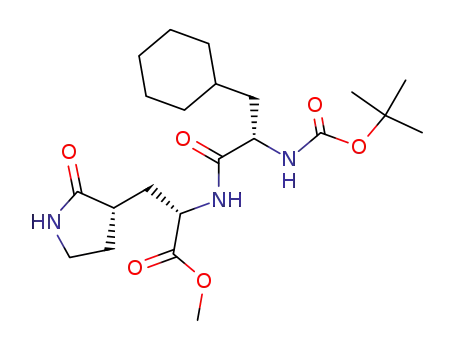
2-(2-tert-butoxycarbonylamino-3-cyclohexyl-propionylamino)-3-(2-oxo-pyrrolidin-3-yl)-propionic acid methyl ester
-
898264-62-5
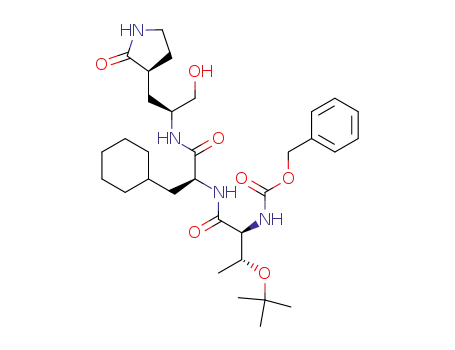
benzyl ((2S,3R)-3-(tert-butoxy)-1-(((S)-3-cyclohexyl-1-(((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)-1-oxopropan-2-yl)amino)-1-oxobutan-2-yl)carbamate
-
898264-61-4
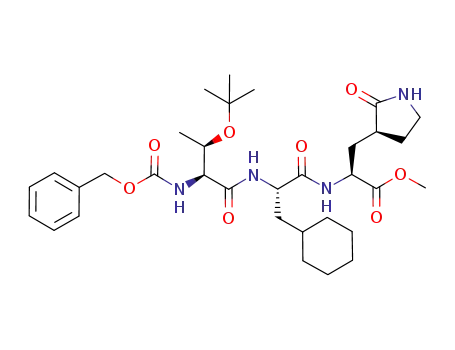
methyl (5S,8S,11S)-5-((R)-1-(tert-butoxy)ethyl)-8-(cyclohexylmethyl)-3,6,9-trioxo-11-(((S)-2-oxopyrrolidin-3-yl)methyl)-1-phenyl-2-oxa-4,7,10-triazadodecan-12-oate
Relevant Products
-
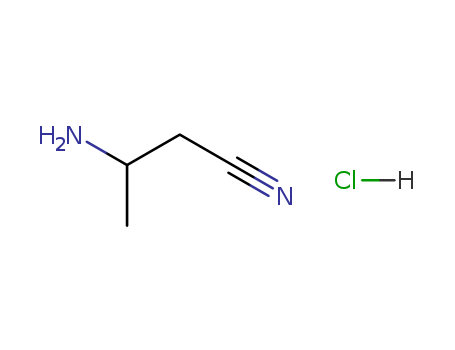
(S)-3-Aminobutyronitrile hydrochloride
CAS:1073666-54-2
-
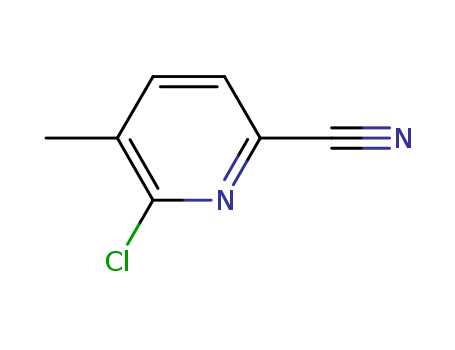
6-Chloro-5-methyl-2-cyanopyridine
CAS:875293-89-3
-
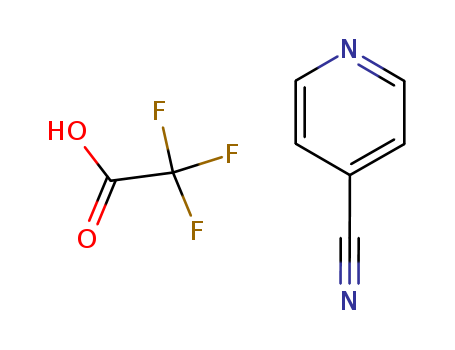
4-cyanopyridine monotrifluoroacetate
CAS:29885-70-9
-
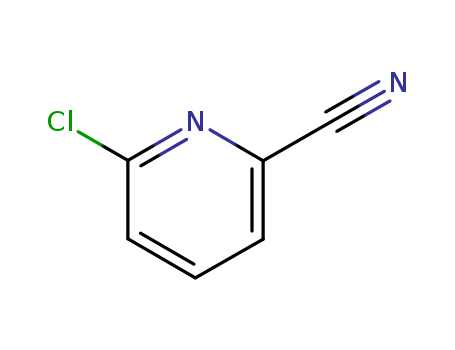
2-Chloro-6-cyanopyridine
CAS:33252-29-8