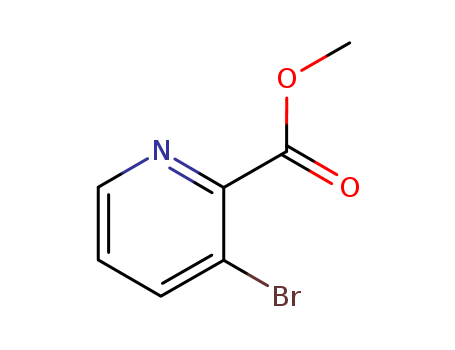
53636-56-9
- Product Name:3-bromo-2-pyridinecarboxylic acid methyl ester
- Molecular Formula:C7H6BrNO2
- Purity:99%
- Molecular Weight:216.034
Product Details
Purity:99%
Reputable supplier selling 3-bromo-2-pyridinecarboxylic acid methyl ester 53636-56-9 with stock
- Molecular Formula:C7H6BrNO2
- Molecular Weight:216.034
- Vapor Pressure:0.008mmHg at 25°C
- Refractive Index:1.554
- Boiling Point:267.423 °C at 760 mmHg
- PKA:-0.91±0.10(Predicted)
- Flash Point:115.533 °C
- PSA:39.19000
- Density:1.58 g/cm3
- LogP:1.63070
methyl 3-bromopicolinate(Cas 53636-56-9) Usage
|
General Description |
Methyl 3-bromopicolinate is a chemical compound with the molecular formula C7H6BrNO2. It is a derivative of pyridine and contains a bromine atom and a methyl ester group. methyl 3-bromopicolinate is often used in organic synthesis as a building block for various pharmaceuticals and agrochemicals. Methyl 3-bromopicolinate is commonly employed as a reagent in Suzuki-Miyaura cross-coupling reactions, which are important in the production of complex organic molecules. Its unique structural properties make it valuable in the development of new compounds with potential applications in medicine and agriculture. Overall, methyl 3-bromopicolinate is a versatile and valuable chemical that plays a crucial role in the field of organic chemistry. |
InChI:InChI=1/C7H6BrNO2/c1-11-7(10)6-5(8)3-2-4-9-6/h2-4H,1H3
53636-56-9 Relevant articles
Palladium NNC Pincer Complex as an Efficient Catalyst for the Cycloisomerization of Alkynoic Acids
Conde, Nerea,SanMartin, Raul,Herrero, María Teresa,Domínguez, Esther
supporting information, p. 3283 - 3292 (2016/10/21)
A two-step (nucleophilic substitution/pa...
NOVEL COMPOUNDS
-
Paragraph 0506-0508, (2015/09/22)
The present invention provides compounds...
Synthesis of regioisomeric pyrido[c]azocanones from azaindanone derivatives
Penning, Miriam,Christoffers, Jens
, p. 2140 - 2149 (2014/04/17)
A ring enlargement reaction with methyla...
Tetrahydropyridinyl and Dihydropyrrolyl Compounds and the Use Thereof
-
Page/Page column 27, (2011/06/24)
The invention relates to tetrahydropyrid...
53636-56-9 Process route
-
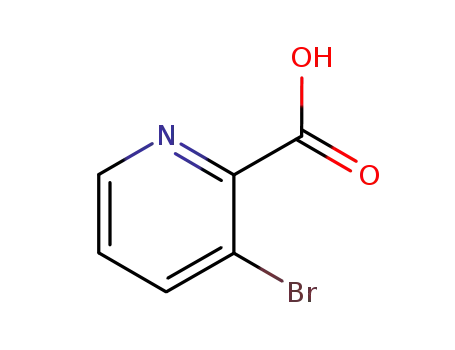
-
30683-23-9
3-bromopicolinic acid

-
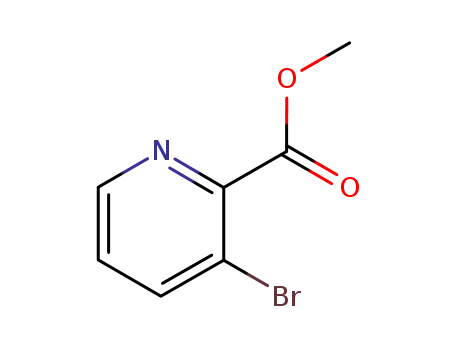
-
53636-56-9
methyl 3-bromopicolinate
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
methanol;
at 20 ℃;
for 12h;
|
82% |
|
Multi-step reaction with 2 steps
1: oxalyl dichloride / dichloromethane; N,N-dimethyl-formamide / 2 h / 20 °C
2: 0 °C
With
oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
|
|
|
With
boron trifluoride;
In
methanol; chloroform;
|
-

-
30683-23-9
3-bromopicolinic acid

-
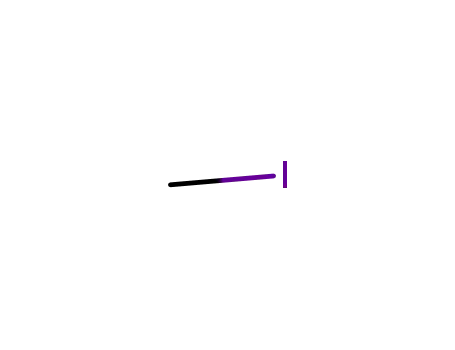
-
74-88-4
methyl iodide

-

-
53636-56-9
methyl 3-bromopicolinate
| Conditions | Yield |
|---|---|
|
3-bromopicolinic acid;
With
sodium hydride;
In
DMF (N,N-dimethyl-formamide);
at 0 ℃;
for 0.833333h;
methyl iodide;
In
DMF (N,N-dimethyl-formamide);
at 0 - 20 ℃;
for 48h;
|
72% |
|
With
sodium hydrogencarbonate;
Yield given. Multistep reaction;
2.) DMF, 0 deg C, 4 h;
|
53636-56-9 Upstream products
-
30683-23-9

3-bromopicolinic acid
-
74-88-4

methyl iodide
-
10354-53-7
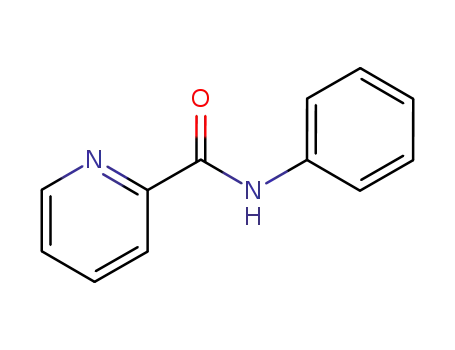
N-phenylpicolinamide
-
188677-47-6
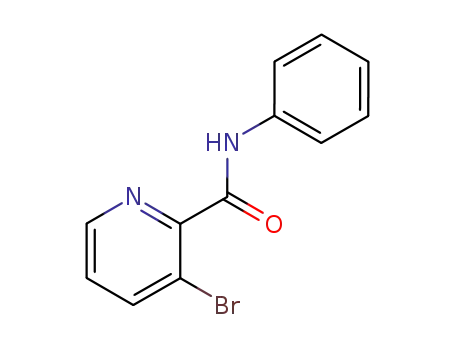
3-Bromo-pyridine-2-carboxylic acid phenylamide
53636-56-9 Downstream products
-
174681-89-1
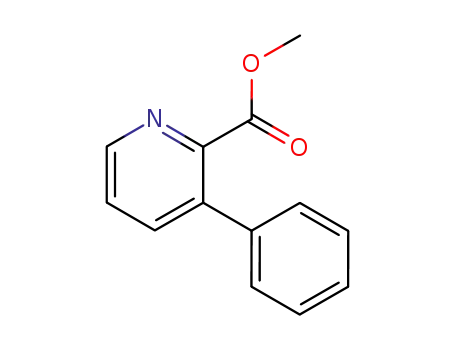
methyl 3-Phenylpyridine-2-carboxylate
-
641627-06-7
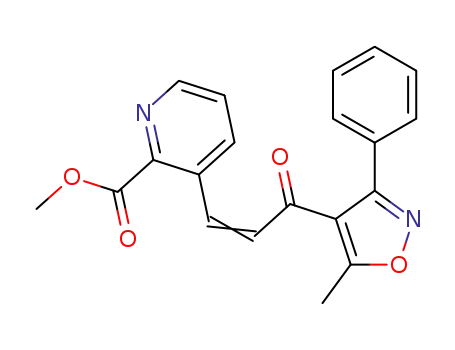
3-[3-(5-methyl-3-phenylisoxazol-4-yl)-3-oxo-propenyl]pyridine-2-carboxylic acid methyl ester
-
54112-52-6
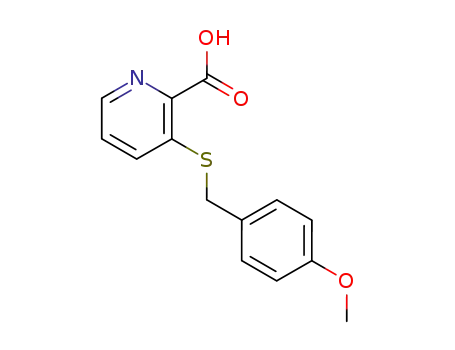
3-(4-methoxy-benzylsulfanyl)-pyridine-2-carboxylic acid
-
1439933-38-6
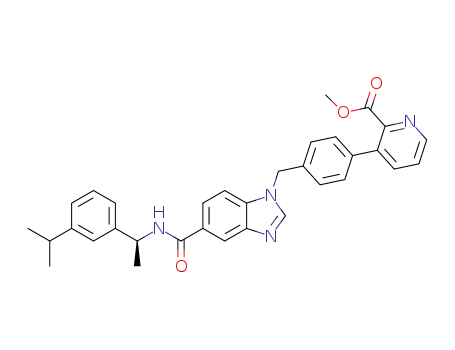
(S)-methyl 3-(4-((5-((1-(3-isopropylphenyl)ethyl)carbamoyl)-1H-benzo[d]imidazol-1-yl)methyl)phenyl)picolinate
Relevant Products
-
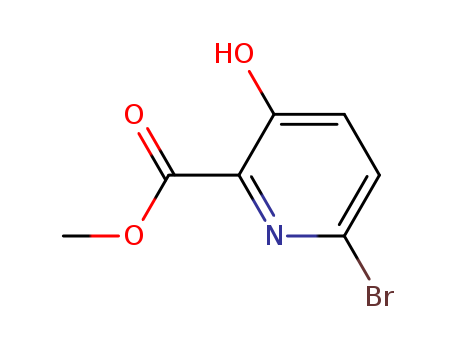
Methyl 6-bromo-3-hydroxy-2-pyridinecarboxylate
CAS:321601-48-3
-
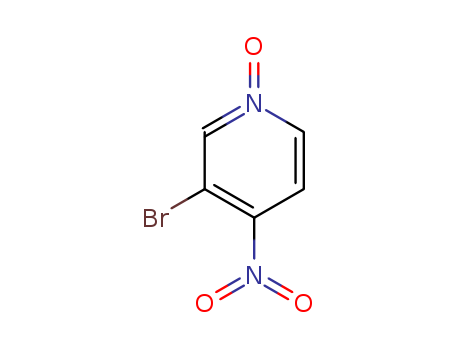
3-bromo-4-nitropyridine oxide
CAS:1678-49-5
-
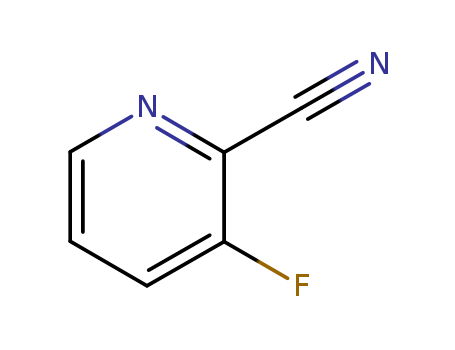
2-cyano-3-fluoropyridine
CAS:97509-75-6