123664-84-6
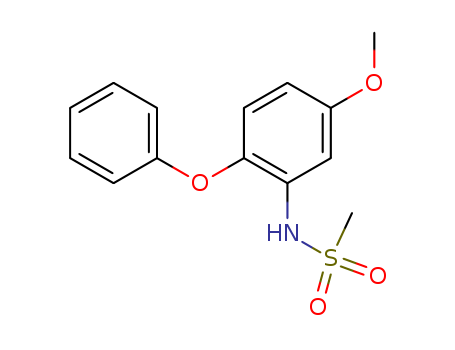
- Product Name:N - (5-methoxy-2-phenoxyphenyl) methanesulfonamide (Imorad intermediate 3)
- Molecular Formula:C14H15NO4S
- Purity:99%
- Molecular Weight:293.343
Product Details
Purity:99%
China cas 123664-84-6 manufacturer wholesale N - (5-methoxy-2-phenoxyphenyl) methanesulfonamide (Imorad intermediate 3) at affordable price
- Molecular Formula:C14H15NO4S
- Molecular Weight:293.343
- Boiling Point:411.4±55.0 °C(Predicted)
- PKA:8.07±0.10(Predicted)
- PSA:73.01000
- Density:1.306±0.06 g/cm3(Predicted)
- LogP:4.01280
Company Profile
Bailong Pharmaceutical officially began operations in 2017, located in the High-tech Zone of Jinan City. It is a high-tech enterprise specializing in the research and production of chemical raw materials and advanced pharmaceutical intermediates, and has currently passed the GB/T 19001-2016/ISO 9001 quality management system certification standards. The company is dedicated to serving major pharmaceutical companies, fine chemical production units, large pharmaceutical research and development centers, and other users of pharmaceutical chemicals both domestically and internationally. It has established cooperation with nearly all of the top ten pharmaceutical companies in the country and hundreds of small and medium-sized biotechnology companies, providing specialized services such as the supply of commercial chemicals, technical research and development, production technology outsourcing, and customized chemicals. In 2020, it was included in the list of "Top Ten Suppliers of the Year" for certain A-share listed companies, reaching a long-term cooperation intention to help clients improve production capacity and development efficiency, and push the project to market at full speed.

Development History
- 2020yearThe application for Shandong high-end pharmaceutical intermediates production base was successful, and it was officially put into operation in June 2022
- 2021yearIn the first quarter, the growth rate reached a new high, with a year-on-year increase of 210%, achieving leapfrog growth in the new fiscal year
- 2022yearIn December,the independent R&D laboratory building of Yinfeng Bio-City was put into operation to help product R&D and innovation.
123664-84-6 Relevant articles
Iguratimod intermediate and synthesis method thereof
-
Paragraph 0036; 0039; 0042, (2019/05/15)
The invention belongs to the technical f...
Synthesis and antiinflammatory activity of 7-methanesulfonylamino-6- phenoxychromones. Antiarthritic effect of the 3-formylamino compound (T-614) in chronic inflammatory disease models
Inaba, Takihiro,Tanaka, Keiichi,Takeno, Ryuko,Nagaki, Hideyoshi,Yoshida, Chosaku,Takano, Shuntaro
, p. 131 - 139 (2007/10/03)
A group of derivatives of 7-methanesulfo...
4H-1-benzopyran-4-one derivative or its salt, process for producing the same and pharmaceutical composition comprising the same as active ingredient
-
, (2008/06/13)
This invention relates to a 4H-1-benzopy...
123664-84-6 Process route
-
- 76838-72-7
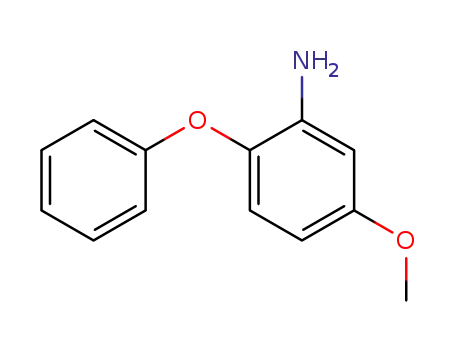
5-methoxy-2-phenoxyaniline

-
- 124-63-0
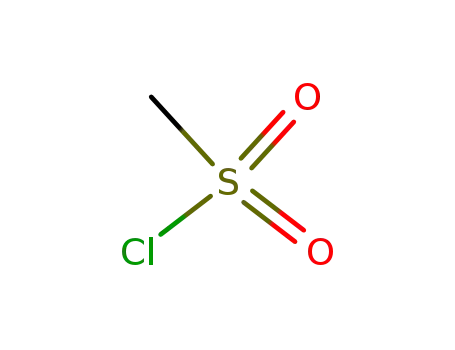
methanesulfonyl chloride

-
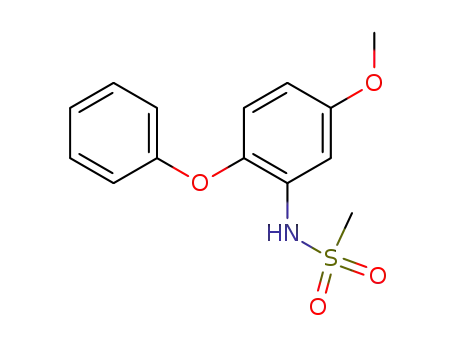
- 123664-84-6
N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In pyridine; water; ethyl acetate;
|
91.9% |
|
With pyridine; at 20 ℃; for 1h;
|
82% |
|
With pyridine; at 5 - 30 ℃; for 1.5h; Temperature; Green chemistry;
|
276.47 g |
-
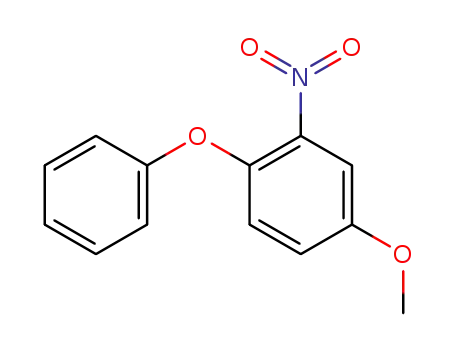
- 84594-95-6
5-methoxy-1-nitro-2-phenoxybenzene

-

- 123664-84-6
N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 72 percent / 4N aq. HCl; Fe / ethanol / 0.33 h / 65 - 70 °C
2: 82 percent / pyridine / 1 h / 20 °C
With pyridine; hydrogenchloride; iron; In ethanol; 1: Reduction / 2: Mesylation;
|
123664-84-6 Upstream products
-
76838-72-7

5-methoxy-2-phenoxyaniline
-
124-63-0

methanesulfonyl chloride
-
84594-95-6

5-methoxy-1-nitro-2-phenoxybenzene
-
10298-80-3
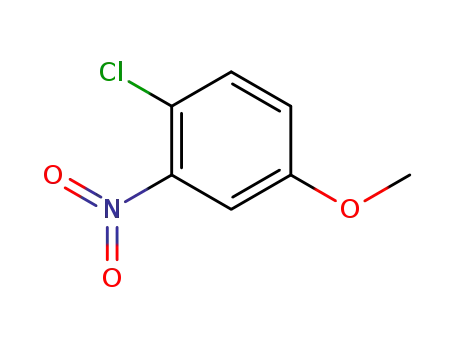
2-chloro-5-methoxynitrobenzene
123664-84-6 Downstream products
-
149436-41-9
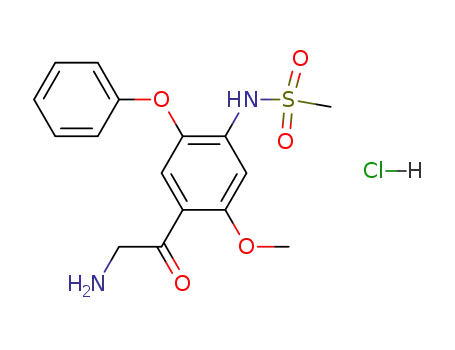
α-amino-2-methoxy-4-methanesulfonamido-5-phenoxyacetophenone hydrochloride
-
123664-55-1
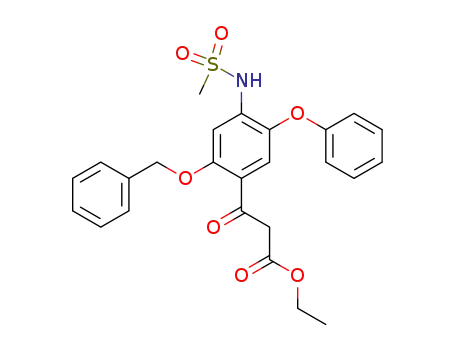
ethyl 2-(2-benzyloxy-4-methylsulfonylamino-5-phenoxybenzoyl)acetate
-
123664-56-2
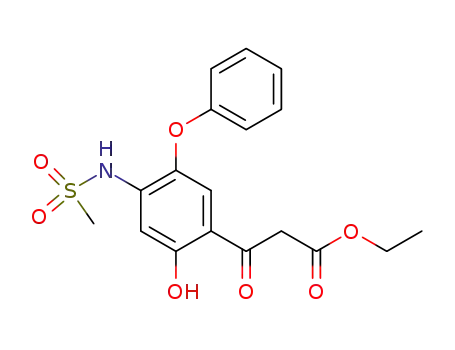
ethyl 3-[2-hydroxy-4-[(methylsulfonyl)amino]-5-phenoxyphenyl]-3-oxopropanoate
-
123663-68-3
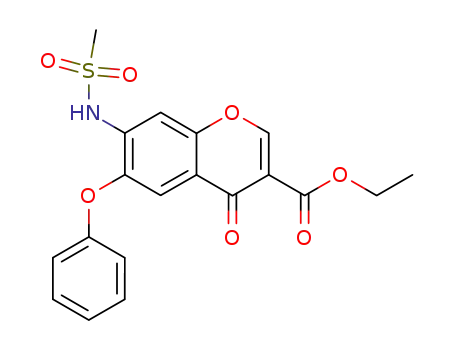
ethyl 7-[(methylsulfonyl)amino]-4-oxo-6-phenoxy-4H-3-chromenecarboxylate
Relevant Products
-
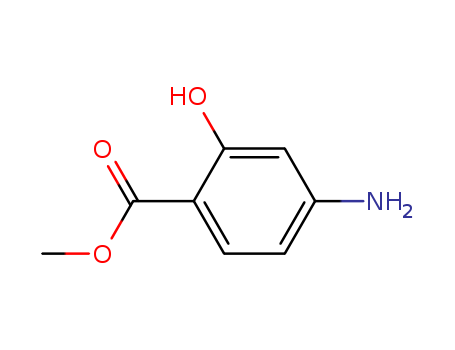
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
-
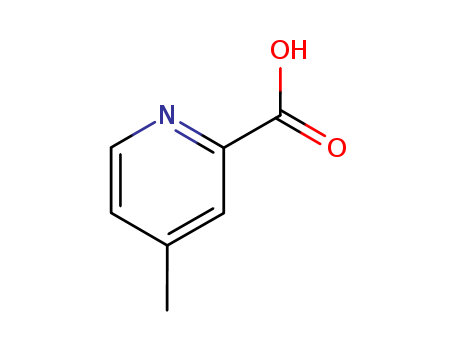
2-Carboxylic acid-4-methylpyridine
CAS:4021-08-3
-
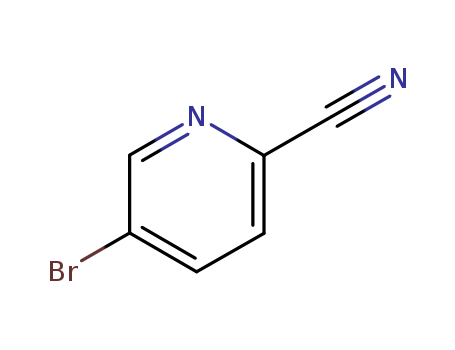
2-cyano-5-bromopyridine
CAS:97483-77-7