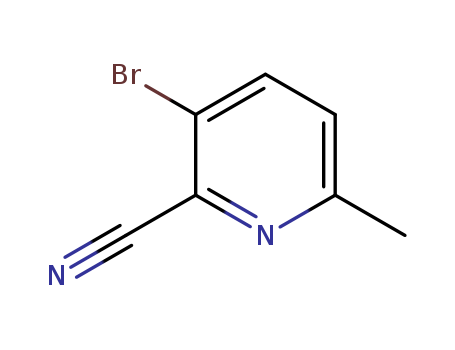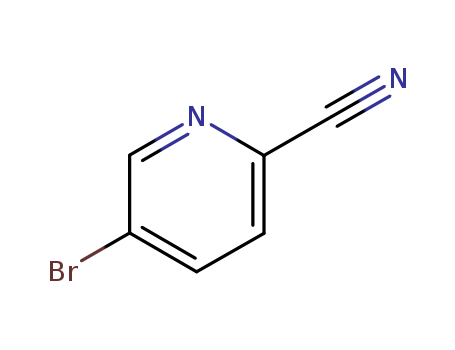
97483-77-7
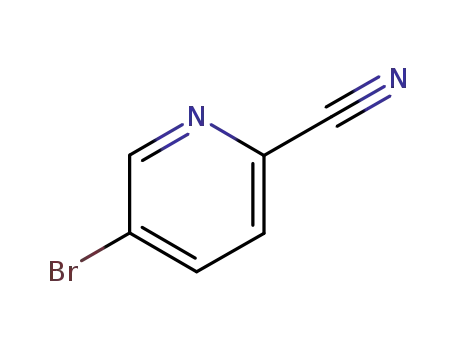
- Product Name:2-cyano-5-bromopyridine
- Molecular Formula:C6H3BrN2
- Purity:99%
- Molecular Weight:183.007
Product Details
pd_meltingpoint:128-132 °C(lit.)
Appearance:light yellow cryst
Purity:99%
Quality Manufacturer Supply High Purity 99% 2-cyano-5-bromopyridine 97483-77-7 with Reasonable Price
- Molecular Formula:C6H3BrN2
- Molecular Weight:183.007
- Appearance/Colour:light yellow cryst
- Vapor Pressure:0.0124mmHg at 25°C
- Melting Point:128-132 °C(lit.)
- Refractive Index:1.611
- Boiling Point:260.3 °C at 760 mmHg
- PKA:-2.68±0.10(Predicted)
- Flash Point:111.2 °C
- PSA:36.68000
- Density:1.72 g/cm3
- LogP:1.71578
5-Bromo-2-pyridinecarbonitrile(Cas 97483-77-7) Usage
InChI:InChI=1/C6H3BrN2/c7-5-1-2-6(3-8)9-4-5/h1-2,4H
97483-77-7 Relevant articles
Preparation method of 2-cyano-5-bromopyridine
-
Paragraph 0035-0038; 0041-0045, (2021/11/06)
The invention provides a preparation met...
2 - Cyano -5 - bromo pyridine preparation method
-
Paragraph 0025-0092, (2019/04/10)
The invention belongs to the field of or...
C?H Cyanation of 6-Ring N-Containing Heteroaromatics
Elbert, Bryony L.,Farley, Alistair J. M.,Gorman, Timothy W.,Johnson, Tarn C.,Genicot, Christophe,Lallemand, Bénédicte,Pasau, Patrick,Flasz, Jakub,Castro, José L.,MacCoss, Malcolm,Paton, Robert S.,Schofield, Christopher J.,Smith, Martin D.,Willis, Michael C.,Dixon, Darren J.
supporting information, p. 14733 - 14737 (2017/10/07)
Heteroaromatic nitriles are important co...
A oxazolidinone compounds of preparation method (by machine translation)
-
Paragraph 0048, (2017/05/12)
The invention provides a oxazolidinone c...
97483-77-7 Process route
-
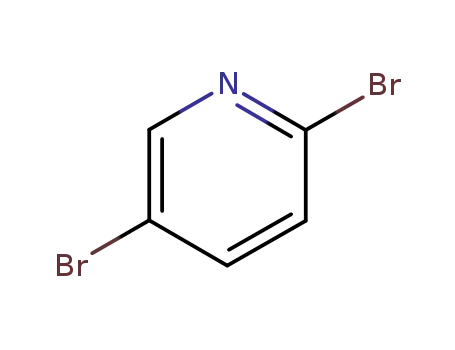
-
624-28-2
2,5-dibromopyridine

-
-
97483-77-7
2-Cyano-5-bromopyridine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: 85 percent / NaI; AcCl / acetonitrile / 3 h / Heating
2.1: iPrMgCl / CH2Cl2; tetrahydrofuran / 0.75 h / 0 °C
2.2: 82 percent / CH2Cl2; tetrahydrofuran / 2 h / 20 °C
With
isopropylmagnesium chloride; acetyl chloride; sodium iodide;
In
tetrahydrofuran; dichloromethane; acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / toluene / 2 h / -78 °C
1.2: toluene / 1 h / -78 °C
2.1: POCl3 / toluene / 5 h / 110 °C
With
n-butyllithium; trichlorophosphate;
In
toluene;
|
|
|
In
N-methyl-acetamide;
|
|
|
With
zinc(II) cyanide; zinc;
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;
In
DMF (N,N-dimethyl-formamide);
for 5h;
Heating / reflux;
|
-

-
624-28-2
2,5-dibromopyridine

-
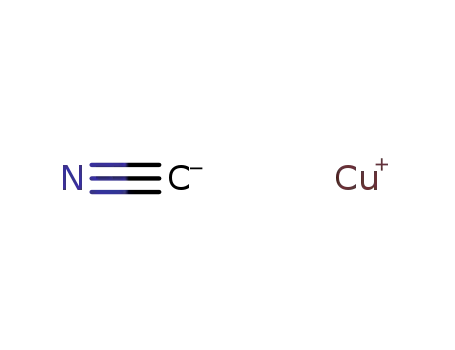
-
copper(l) cyanide

-

-
97483-77-7
2-Cyano-5-bromopyridine
| Conditions | Yield |
|---|---|
|
With
sodium cyanide;
In
N,N-dimethyl-formamide;
at 150 ℃;
for 7h;
Inert atmosphere;
|
70% |
97483-77-7 Upstream products
-
2402-97-3
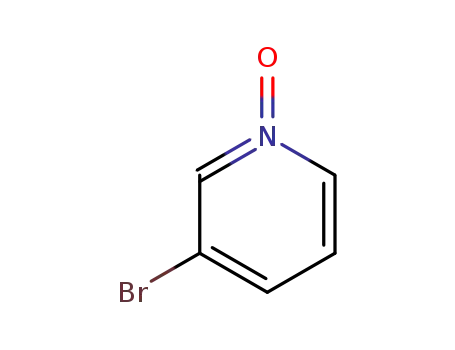
3-bromopyridine N-oxide
-
7677-24-9
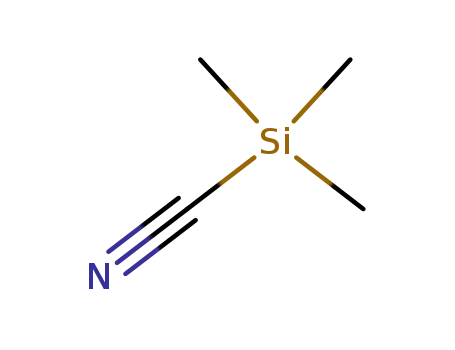
trimethylsilyl cyanide
-
647826-69-5
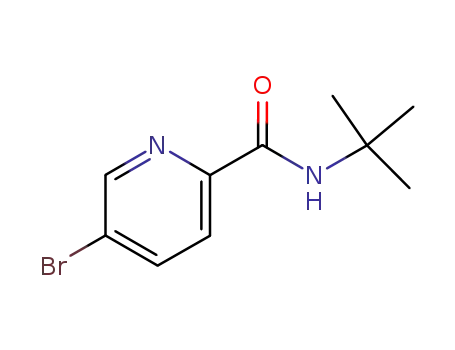
5-bromo-pyridine-2-carboxylic acid tert-butylamide
-
624-28-2

2,5-dibromopyridine
97483-77-7 Downstream products
-
380380-60-9
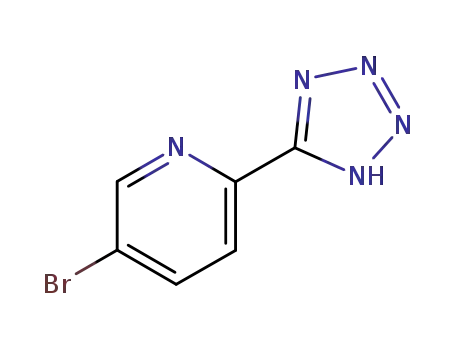
2-(1,2,3,4-tetrazol-5-yl)-5-bromopyridine
-
741709-63-7
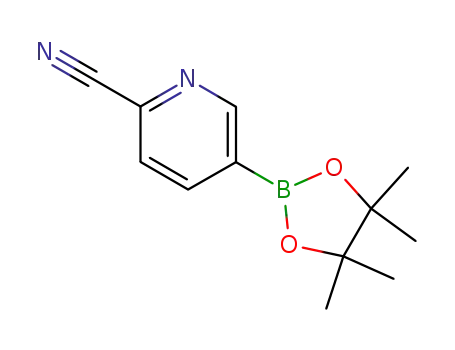
2-cyanopyridine-5-boronic acid pinacol ester
-
30766-11-1
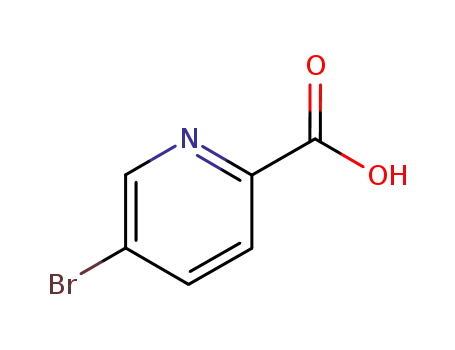
5-bromoisonicotinic acid
-
944718-22-3
1-(5-bromopyridin-2-yl)cyclopropanamine
Relevant Products
-
3-bromo-6-methylpyridine-2-carboxylic acid
CAS:717843-48-6
-
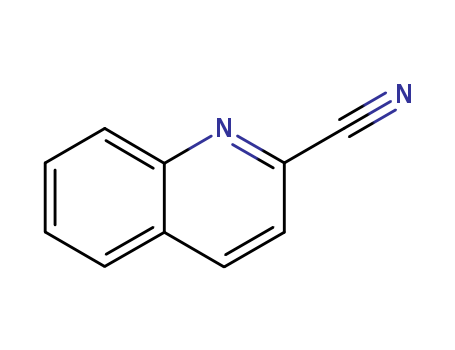
2-cyanoquinoline
CAS:1436-43-7