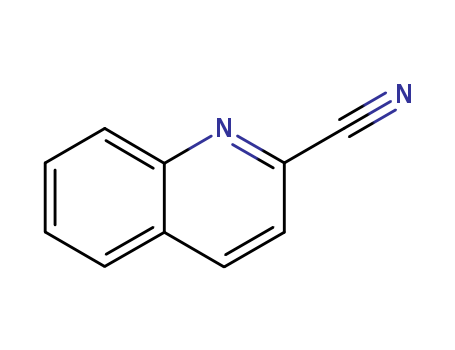
1436-43-7
- Product Name:2-cyanoquinoline
- Molecular Formula:C10H6N2
- Purity:99%
- Molecular Weight:154.171
Product Details
pd_meltingpoint:93-95 °C(lit.)
Purity:99%
Cosmetics Grade 2-cyanoquinoline 1436-43-7 For Sale with Good Price
- Molecular Formula:C10H6N2
- Molecular Weight:154.171
- Vapor Pressure:0.000258mmHg at 25°C
- Melting Point:93-95 °C(lit.)
- Refractive Index:1.654
- Boiling Point:323.69 °C at 760 mmHg
- PKA:-0.56±0.40(Predicted)
- Flash Point:115.537 °C
- PSA:36.68000
- Density:1.211 g/cm3
- LogP:2.10648
QUINOLINE-2-CARBONITRILE(Cas 1436-43-7) Usage
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 81, p. 4004, 1959 DOI: 10.1021/ja01524a046 |
|
General Description |
Mechanism of the photoinduced substitution reaction of 2-quinolinecarbonitrile in alcohols or ethers has been reported. Photoinitiated dimerization of 2-quinolinecarbonitrile in HCl-acidified 2-propanol/water has been investigated at 77K and 331K. Benzophenone-sensitization of 2-quinolinecarbonitrile has been reported to yield triazapentaphene. |
InChI:InChI=1/C10H6N2/c11-7-9-6-5-8-3-1-2-4-10(8)12-9/h1-6H
1436-43-7 Relevant articles
Corrigendum: Organo-Photoredox Catalyzed Oxidative Dehydrogenation of N-Heterocycles (Chemistry - A European Journal, (2017), 23, 57, (14167-14172), 10.1002/chem.201703642)
Sahoo, Manoj K.,Jaiswal, Garima,Rana, Jagannath,Balaraman, Ekambaram
, p. 7038 - 7038 (2019)
The authors have been alerted to an erro...
(Benzo[h])quinolinyl-substituted monoazatriphenylenes: Synthesis and photophysical properties
Kopchuk,Khasanov,Kovalev,Kim,Nikonov,Zyryanov,Rusinov,Chupakhin
, p. 864 - 870 (2014)
We propose a method for the synthesis of...
Structure-function studies on a synthetic guanosine receptor that simultaneously binds Watson-Crick and Hoogsteen sites
Quinn, Jordan R.,Zimmerman, Steven C.
, p. 7459 - 7467 (2005)
A series of receptors (11-16) designed t...
Rearrangements of 4-Quinolylcarbene, 3-Quinolylcarbene, and 2-Quinolylcarbene to 1-Naphthylnitrene and Cyanoindenes by Falling Solid Flash Vacuum Pyrolysis
Aylward, Nigel,Kvaskoff, David,Becker, Jürgen,Wentrup, Curt
, p. 4609 - 4615 (2016)
The relationship between 4-quinolylcarbe...
-
Hata et al.
, p. 2286 (1971)
-
One-pot synthesis of nitriles from aldehydes and hydroxylamine hydrochloride over silica gel, montmorillonites K-10, and KSF catalysts in dry media under microwave irradiation
Dewan, Sharwan K.,Singh, Ravinder,Kumar, Anil
, p. 2025 - 2029 (2004)
A rapid and facile one-pot synthesis of ...
Regioselective direct oxidative C-H cyanation of quinoline and its derivatives catalyzed by vanadium-containing heteropoly acids
Yamaguchi, Kazuya,Xu, Ning,Jin, Xiongjie,Suzuki, Kosuke,Mizuno, Noritaka
, p. 10034 - 10037 (2015)
A direct oxidative C-H cyanation of quin...
Triselenium dicyanide (TSD) as a new cyanation reagent: Synthesis of cyano pterins and quinoxalines along with library of cyano N-heterocyclic compounds
Goswami, Shyamaprosad,Maity, Annada C.,Das, Nirmal K.,Sen, Debabrata,Maity, Sibaprasad
, p. 407 - 415 (2009)
Triselenium dicynide (TSD) has been repo...
Highly chemoselective deoxygenation of N-heterocyclic: N -oxides under transition metal-free conditions
Kim, Se Hyun,An, Ju Hyeon,Lee, Jun Hee
supporting information, p. 3735 - 3742 (2021/05/04)
Because their site-selective C-H functio...
Metal-Free Deoxygenation of Amine N-Oxides: Synthetic and Mechanistic Studies
Lecroq, William,Schleinitz, Jules,Billoue, Mallaury,Perfetto, Anna,Gaumont, Annie-Claude,Lalevée, Jacques,Ciofini, Ilaria,Grimaud, Laurence,Lakhdar, Sami
, p. 1237 - 1242 (2021/06/01)
We report herein an unprecedented combin...
Cascade Process for Direct Transformation of Aldehydes (RCHO) to Nitriles (RCN) Using Inorganic Reagents NH2OH/Na2CO3/SO2F2 in DMSO
Fang, Wan-Yin,Qin, Hua-Li
, p. 5803 - 5812 (2019/05/14)
A simple, mild, and practical process fo...
Regioselective Cyanation of Six-Membered N-Heteroaromatic Compounds Under Metal-, Activator-, Base- and Solvent-Free Conditions
Sarmah, Bikash Kumar,Konwar, Monuranjan,Bhattacharyya, Dipanjan,Adhikari, Priyanka,Das, Animesh
supporting information, p. 5616 - 5625 (2019/11/22)
A regioselective cyanation of heteroarom...
1436-43-7 Process route

-
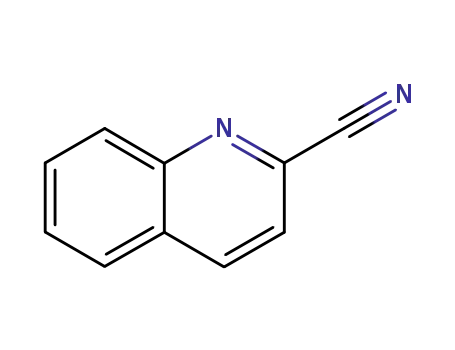
-
1436-43-7
quinoline-2-carbonitrile

-
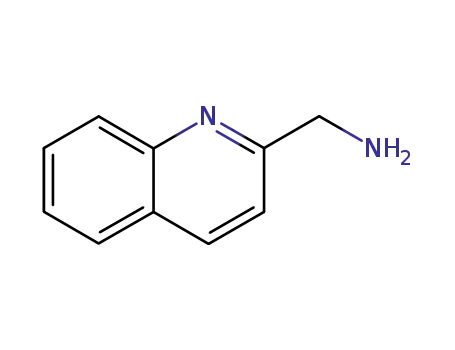
-
5760-20-3
2-aminomethylquinoline
| Conditions | Yield |
|---|---|
|
|
88% |
-
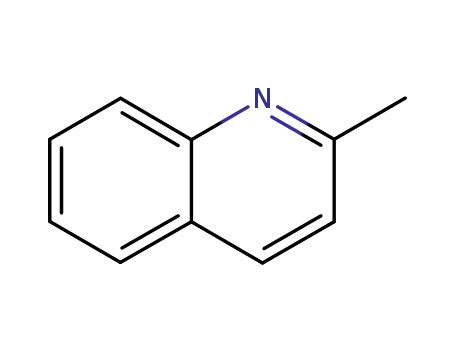
-
91-63-4
2-methylquinoline

-
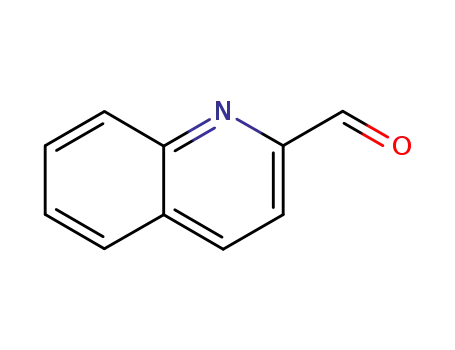
-
5470-96-2
quinoline 2-carbaldehyde

-

-
1436-43-7
quinoline-2-carbonitrile

-
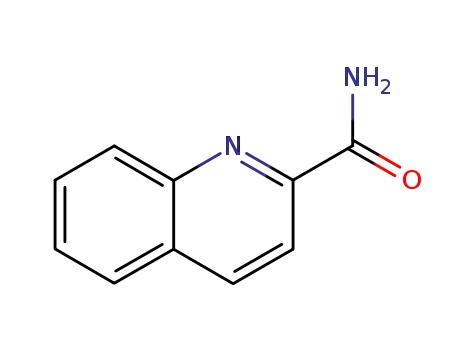
-
5382-42-3
quinoline-2-carboxamide
| Conditions | Yield |
|---|---|
|
With
manganese(IV) oxide; oxygen; urea;
at 150 ℃;
for 3h;
under 3800.26 Torr;
Autoclave;
|
81 %Chromat. 16 %Chromat. 48 %Chromat. |
1436-43-7 Upstream products
-
830-60-4
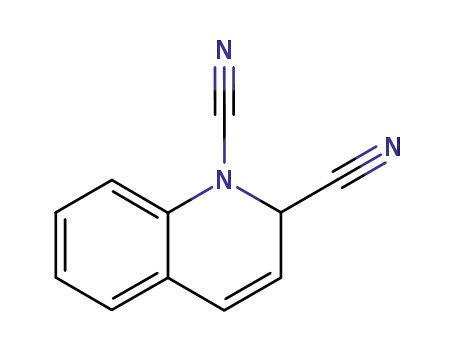
2H-quinoline-1,2-dicarbonitrile
-
612-62-4
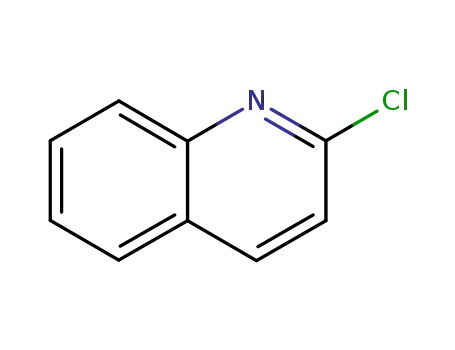
2-Chloroquinoline
-
74-90-8

hydrogen cyanide
-
1613-37-2
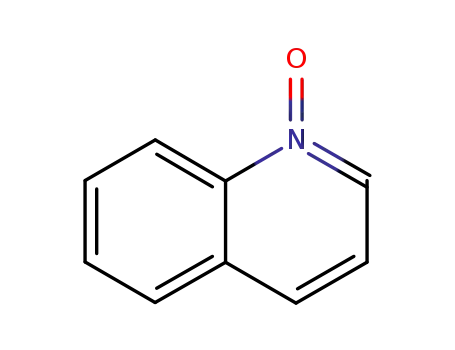
Quinoline N-oxide
1436-43-7 Downstream products
-
1011-47-8
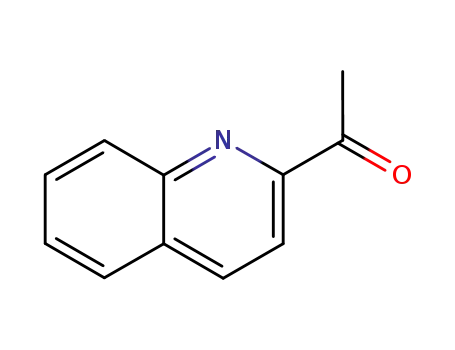
1-quinolin-2-yl-ethanone
-
860717-00-6
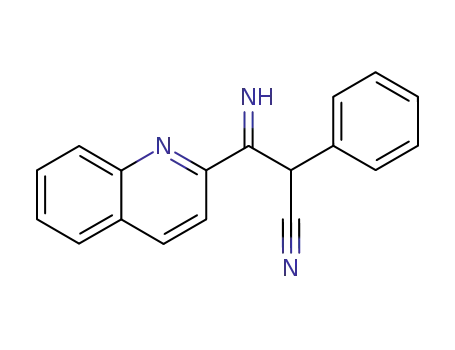
3-[2]quinolyl-3-imino-2-phenyl-propionitrile
-
16576-25-3
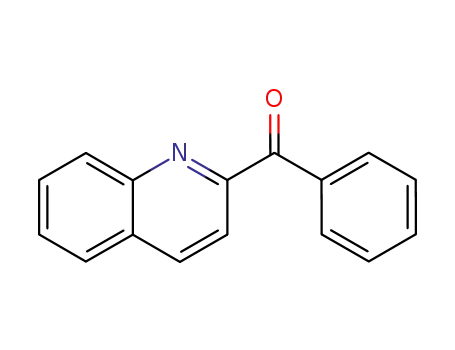
2-benzoylquinoline
-
10421-37-1
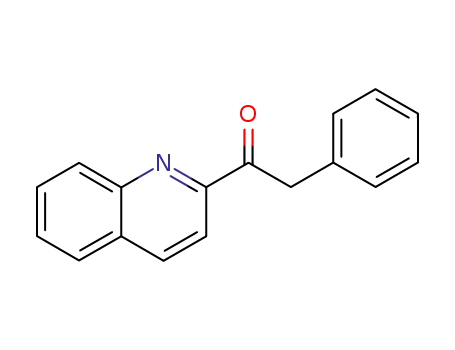
1-[2]quinolyl-2-phenyl-ethanone
Relevant Products
-
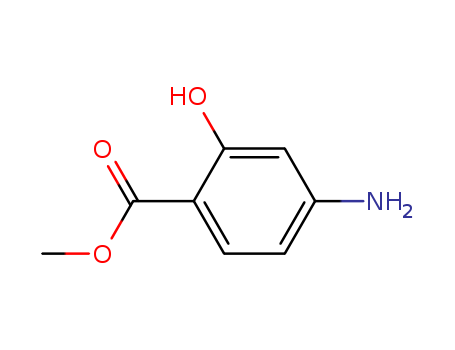
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
-
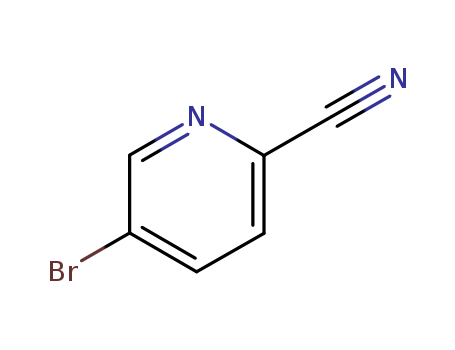
2-cyano-5-bromopyridine
CAS:97483-77-7
-
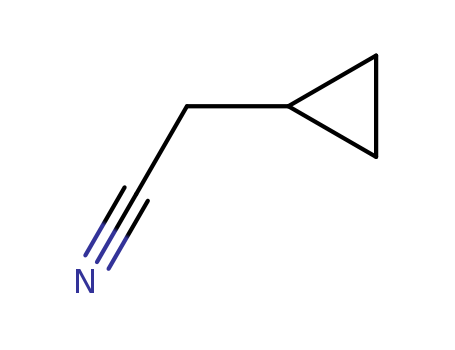
Cyclopropaneacetonitrile
CAS:6542-60-5