152126-31-3
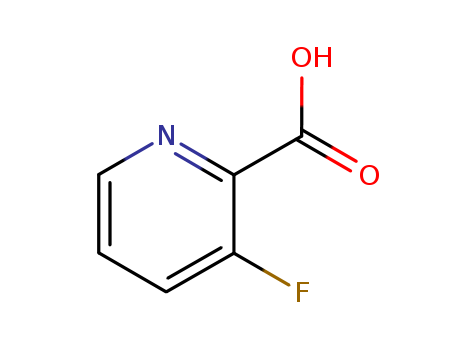
- Product Name:3-Fluoropyridine-2-carboxylic acid
- Molecular Formula:C6H4FNO2
- Purity:99%
- Molecular Weight:141.102
Product Details
pd_meltingpoint:154 °C
Purity:99%
Excellent chemical plant bulk supply 3-Fluoropyridine-2-carboxylic acid 152126-31-3
- Molecular Formula:C6H4FNO2
- Molecular Weight:141.102
- Vapor Pressure:0.00465mmHg at 25°C
- Melting Point:154 °C
- Refractive Index:1.541
- Boiling Point:265.2 °C at 760 mmHg
- PKA:2.71±0.10(Predicted)
- Flash Point:114.2 °C
- PSA:50.19000
- Density:1.419 g/cm3
- LogP:0.91890
3-FLUOROPYRIDINE-2-CARBOXYLIC ACID(Cas 152126-31-3) Usage
|
General Description |
3-Fluoropyridine-2-Carboxylic Acid is a fluorinated pyridine derivative. It is often used in scientific research, particularly in the field of organic chemistry, as a building block for the synthesis of more complex compounds. This chemical compound has unique properties due to the presence of a fluorine atom and carboxylic acid group. Its structure comprises a pyridine ring, a type of aromatic heterocyclic compound, with a fluorine atom attached at the 3-position and a carboxylic acid group at the 2-position. It is generally available as a light yellow to brown crystalline powder and has the molecular formula of C6H4FNO2. |
InChI:InChI=1/C6H4FNO2/c7-4-2-1-3-8-5(4)6(9)10/h1-3H,(H,9,10)
152126-31-3 Relevant articles
The discovery of potent small molecule cyclic urea activators of STING
Banerjee, Monali,Basu, Sourav,Ghosh, Rajib,Middya, Sandip,Pryde, David C.,Shrivastava, Ritesh,Surya, Arjun,Yadav, Dharmendra B.
supporting information, (2022/02/07)
STING mediates innate immune responses t...
Synthesis, antimycobacterial activity and influence on mycobacterial InhA and PknB of 12-membered cyclodepsipeptides
Laqua, Katja,Klemm, Marcel,Richard-Greenblatt, Melissa,Richter, Adrian,Liebe, Linda,Huang, Tingting,Lin, Shuangjun,Guardia, Ana,Pérez-Herran, Esther,Ballell, Lluís,Av-Gay, Yossef,Imming, Peter
, p. 3166 - 3190 (2018/05/05)
In recent years, several small natural c...
SPECIFIC CARBOXAMIDES AS KCNQ2/3 MODULATORS
-
Page/Page column 38, (2014/06/23)
The invention relates to specific carbox...
Converting core compounds into building blocks: The concept of regiochemically exhaustive functionalization
Marzi, Elena,Bobbio, Carla,Cottet, Fabrice,Schlosser, Manfred
, p. 2116 - 2123 (2007/10/03)
In a model study, 3-fluorophenol and 3-f...
152126-31-3 Process route
-
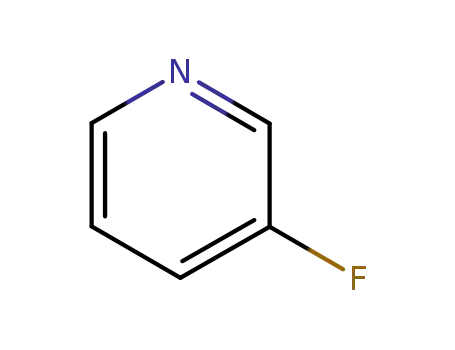
- 372-47-4
3-Fluoropyridine

-
- 124-38-9,18923-20-1
carbon dioxide

-
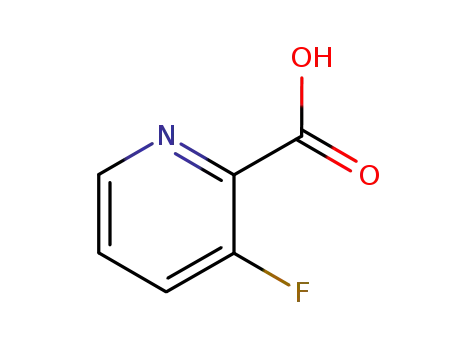
- 152126-31-3
3-fluoro(pyridin-2-yl)carboxylic acid
| Conditions | Yield |
|---|---|
|
With 1,4-diaza-bicyclo[2.2.2]octane; n-butyllithium; In diethyl ether; hexane; at -75 ℃;
|
49% |
|
3-Fluoropyridine; With 1,4-diaza-bicyclo[2.2.2]octane; n-butyllithium; In diethyl ether; hexane; at -78 - -60 ℃; for 1h;
carbon dioxide; In diethyl ether; hexane; at -78 - -10 ℃; for 0.333333h;
|
1.2 g |
-
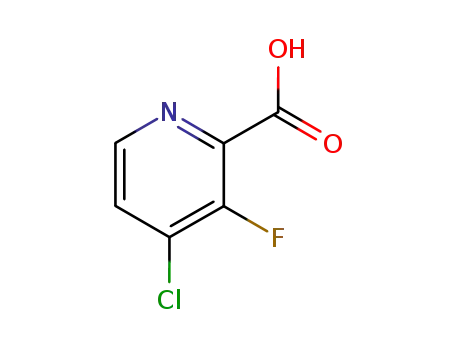
- 860296-21-5
4-chloro-3-fluoropyridine-2-carboxylic acid

-

- 152126-31-3
3-fluoro(pyridin-2-yl)carboxylic acid
| Conditions | Yield |
|---|---|
|
With hydrogen; ammonium formate; palladium on activated charcoal; In ethanol; for 6h;
|
89% |
152126-31-3 Upstream products
-
372-47-4

3-Fluoropyridine
-
124-38-9

carbon dioxide
-
860296-21-5

4-chloro-3-fluoropyridine-2-carboxylic acid
-
2546-56-7
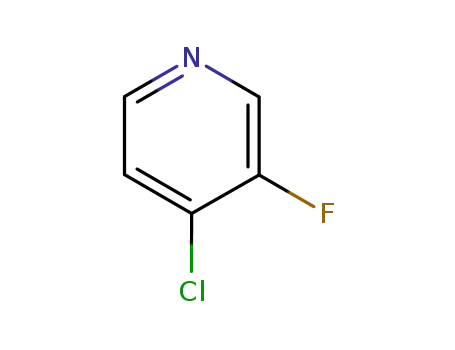
4-chloro-3-fluoro-pyridine
152126-31-3 Downstream products
-
1086562-12-0
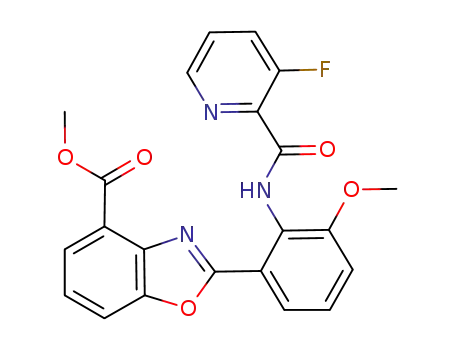
2-[2-[(3-fluoropyridine-2-carbonyl)-amino]-3-methoxyphenyl]-benzoxazole-4-carboxylic acid methyl ester
-
1254364-39-0
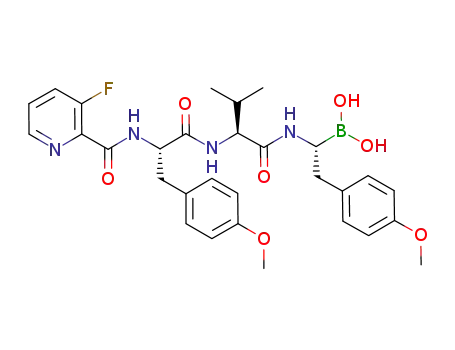
C30H36BFN4O7
-
886510-21-0
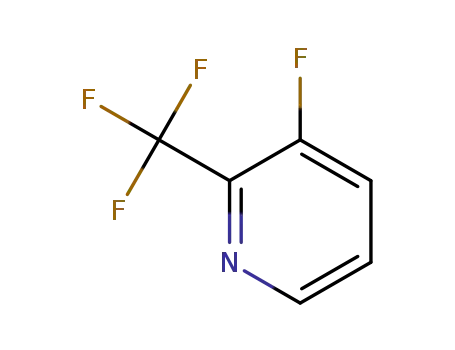
3-fluoro-2-(trifluoromethyl)pyridine
-
869108-35-0
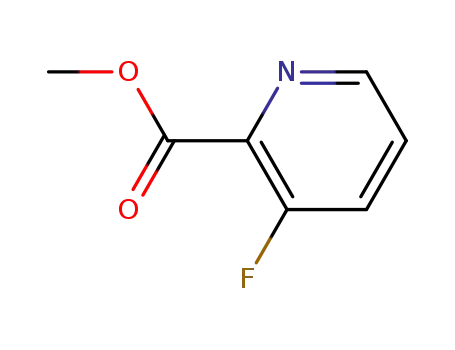
methyl 3-fluoropyridine-2-carboxylate
Relevant Products
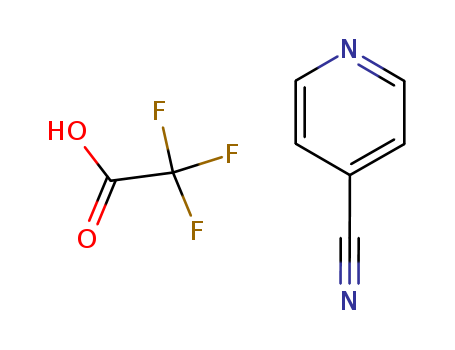
-
4-cyanopyridine monotrifluoroacetate
CAS:29885-70-9
-
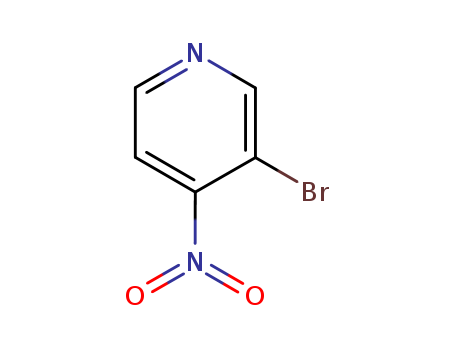
3-bromo-4-nitropyridine
CAS:89364-04-5
-
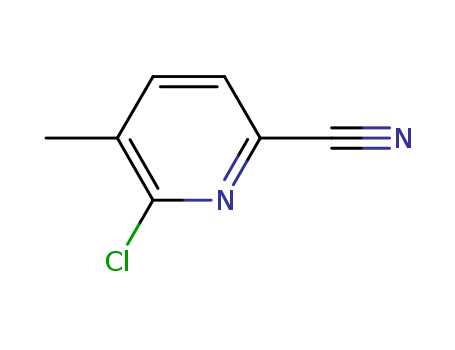
6-Chloro-5-methyl-2-cyanopyridine
CAS:875293-89-3