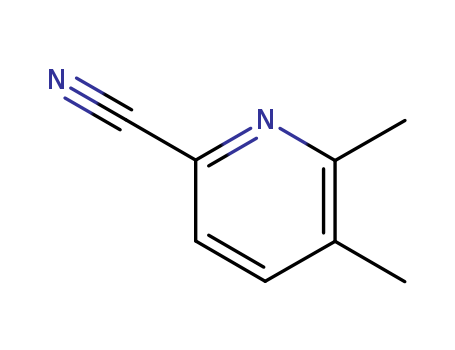
59146-67-7
- Product Name:2-Cyano-5,6-dimethylpyridine
- Molecular Formula:C8H8N2
- Purity:99%
- Molecular Weight:132.16
Product Details
Purity:99%
Purity 99% Min 2-Cyano-5,6-dimethylpyridine 59146-67-7 Spot Supply with Safe Transportation
- Molecular Formula:C8H8N2
- Molecular Weight:132.16
- Boiling Point:280.7±35.0 °C(Predicted)
- PKA:0.69±0.10(Predicted)
- PSA:36.68000
- Density:1.05±0.1 g/cm3(Predicted)
- LogP:1.57008
Company Profile
Bailong Pharmaceutical officially began operations in 2017, located in the High-tech Zone of Jinan City. It is a high-tech enterprise specializing in the research and production of chemical raw materials and advanced pharmaceutical intermediates, and has currently passed the GB/T 19001-2016/ISO 9001 quality management system certification standards. The company is dedicated to serving major pharmaceutical companies, fine chemical production units, large pharmaceutical research and development centers, and other users of pharmaceutical chemicals both domestically and internationally. It has established cooperation with nearly all of the top ten pharmaceutical companies in the country and hundreds of small and medium-sized biotechnology companies, providing specialized services such as the supply of commercial chemicals, technical research and development, production technology outsourcing, and customized chemicals. In 2020, it was included in the list of "Top Ten Suppliers of the Year" for certain A-share listed companies, reaching a long-term cooperation intention to help clients improve production capacity and development efficiency, and push the project to market at full speed.

Development History
- 2020yearThe application for Shandong high-end pharmaceutical intermediates production base was successful, and it was officially put into operation in June 2022
- 2021yearIn the first quarter, the growth rate reached a new high, with a year-on-year increase of 210%, achieving leapfrog growth in the new fiscal year
- 2022yearIn December,the independent R&D laboratory building of Yinfeng Bio-City was put into operation to help product R&D and innovation.
59146-67-7 Relevant articles
SUBSTITUTED PYRIDIZINONE DERIVATIVES AS PDE10 INHIBITORS
-
Page/Page column 57, (2014/09/29)
The present invention is directed to sub...
SUBSTITUTED PYRIDIZINONE DERIVATIVES AS PDE10 INHIBITORS
-
Page/Page column 57, (2014/10/04)
The present invention is directed to sub...
5-lipoxygenase-activating protein (FLAP) inhibitors. Part 4: Development of 3-[3-tert-butylsulfanyl-1-[4-(6-ethoxypyridin-3-yl)benzyl]-5-(5-methylpyridin- 2-ylmethoxy)-1 H -indol-2-yl]-2,2-dimethylpropionic acid (AM803), a potent, oral, once daily FLAP inhibitor
Stock, Nicholas S.,Bain, Gretchen,Zunic, Jasmine,Li, Yiwei,Ziff, Jeannie,Roppe, Jeffrey,Santini, Angelina,Darlington, Janice,Prodanovich, Pat,King, Christopher D.,Baccei, Christopher,Lee, Catherine,Rong, Haojing,Chapman, Charles,Broadhead, Alex,Lorrain, Dan,Correa, Lucia,Hutchinson, John H.,Evans, Jilly F.,Prasit, Peppi
experimental part, p. 8013 - 8029 (2012/03/08)
The potent 5-lipoxygenase-activating pro...
BENZOXAZINONE DERIVATIVES FOR THE TREATMENT OF GLYTL MEDIATED DISORDERS
-
Page/Page column 63; 64, (2011/02/24)
The present invention relates to benzoxa...
59146-67-7 Process route
-
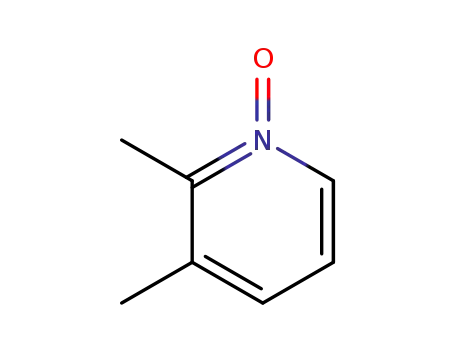
- 22710-07-2,166521-76-2
2,3-dimethylpyridine 1-oxide

-
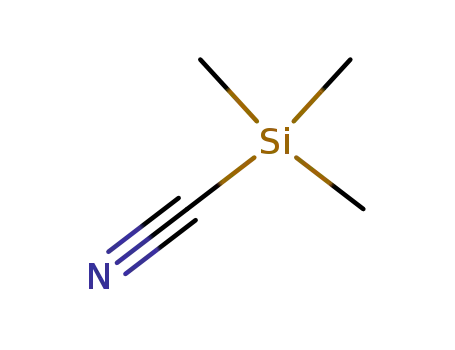
- 7677-24-9
trimethylsilyl cyanide

-
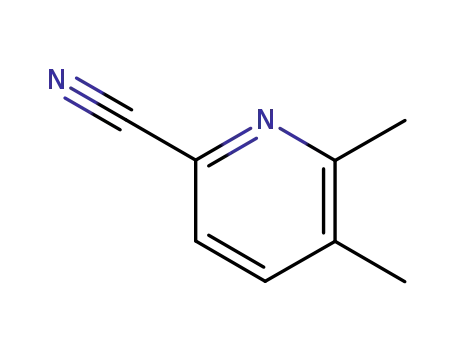
- 59146-67-7
5,6-dimethylpyridine-2-carbonitrile
| Conditions | Yield |
|---|---|
|
With N,N-Dimethylcarbamoyl chloride; In dichloromethane; at 20 ℃; for 72h;
|
75% |
|
With N,N-diethylcarbamyl chloride; In dichloromethane; at 20 ℃; for 60h; Inert atmosphere;
|
53% |
|
With N,N-diethylcarbamyl chloride; In 1,2-dichloro-ethane; Ambient temperature;
|
|
|
2,3-dimethylpyridine 1-oxide; trimethylsilyl cyanide; With triethylamine; In acetonitrile; at 90 ℃; for 120h; Inert atmosphere;
With sodium hydroxide; In water; acetonitrile; at 0 ℃;
|
|
|
2,3-dimethylpyridine 1-oxide; trimethylsilyl cyanide; In dichloromethane; at 20 ℃; for 0.5h;
With N,N-diethylcarbamyl chloride; In dichloromethane; at 20 ℃; for 24h;
|
|
|
2,3-dimethylpyridine 1-oxide; trimethylsilyl cyanide; In dichloromethane; at 20 ℃; for 0.5h;
With N,N-diethylcarbamyl chloride; In dichloromethane; for 24h;
|
-
aq. K2 CO3
-
aq. K2 CO3

-
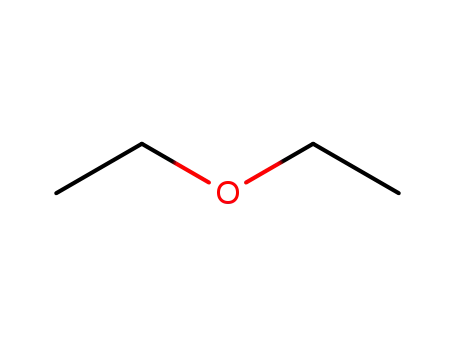
- 60-29-7,927820-24-4
diethyl ether

-
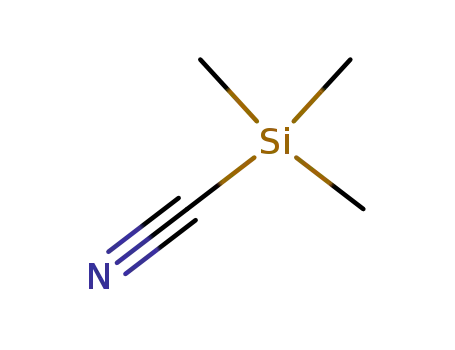
- 7677-24-9
trimethylsilyl cyanide

-
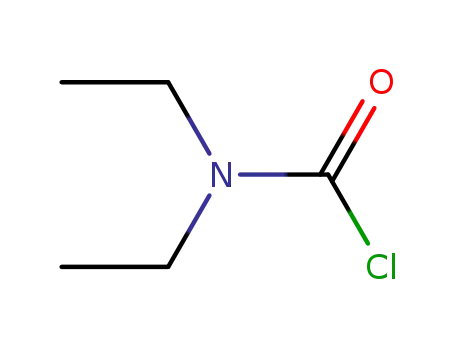
- 88-10-8
N,N-diethylcarbamyl chloride

-

- 59146-67-7
5,6-dimethylpyridine-2-carbonitrile
| Conditions | Yield |
|---|---|
|
In dichloromethane;
|
59146-67-7 Upstream products
-
22710-07-2

2,3-dimethylpyridine 1-oxide
-
7677-24-9
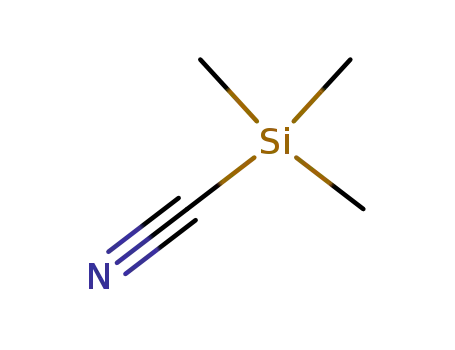
trimethylsilyl cyanide
-
60-29-7

diethyl ether
-
88-10-8

N,N-diethylcarbamyl chloride
59146-67-7 Downstream products
-
543713-56-0
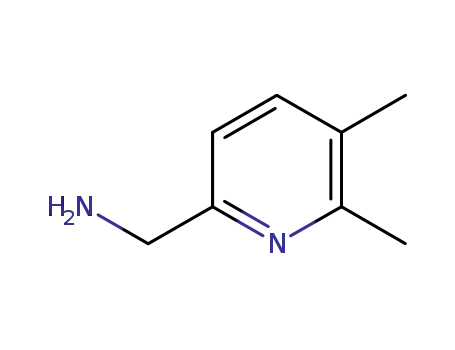
(5,6-dimethylpyridin-2-yl)methanamine
-
153646-65-2
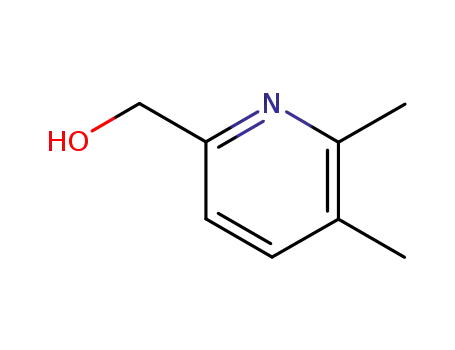
2-hydroxymethyl-5,6-dimethylpyridine
-
83282-49-9
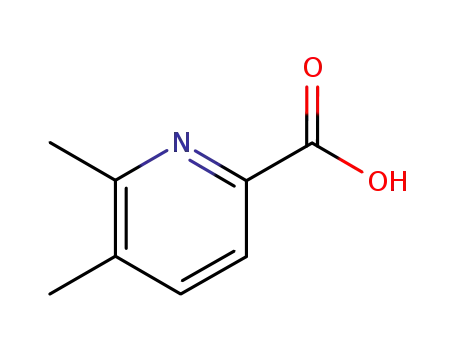
5,6-dimethyl-pyridine-2-carboxylic acid
Relevant Products
-
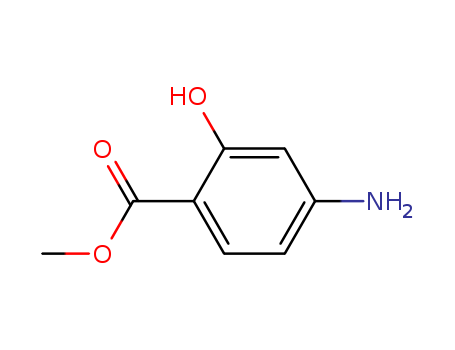
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
-
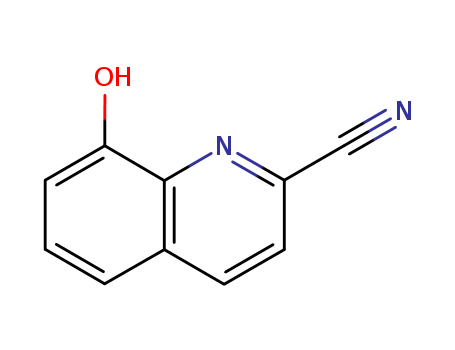
8-Hydroxyquinoline-2-carboxylic acid
CAS:6759-78-0
-
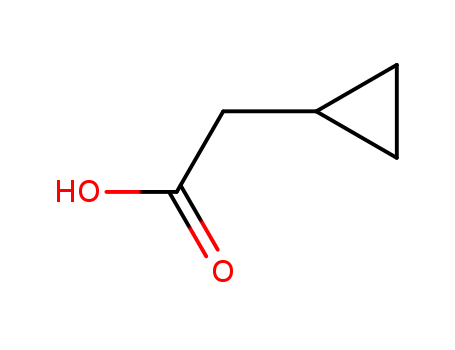
Cyclopropaneacetic acid
CAS:5239-82-7