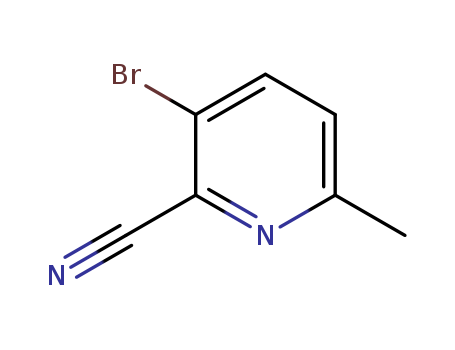
717843-48-6
- Product Name:3-bromo-6-methylpyridine-2-carboxylic acid
- Molecular Formula:C7H5BrN2
- Purity:99%
- Molecular Weight:197.034
Product Details
Purity:99%
Quality Manufacturer Supply High Purity 99% 3-bromo-6-methylpyridine-2-carboxylic acid 717843-48-6 with Reasonable Price
- Molecular Formula:C7H5BrN2
- Molecular Weight:197.034
- Vapor Pressure:0.001mmHg at 25°C
- Refractive Index:1.594
- Boiling Point:299.304 °C at 760 mmHg
- PKA:-1.95±0.10(Predicted)
- Flash Point:134.815 °C
- PSA:36.68000
- Density:1.613 g/cm3
- LogP:2.02418
3-Bromo-2-cyano-6-methylpyridine(Cas 717843-48-6) Usage
|
General Description |
3-Bromo-2-cyano-6-methylpyridine is a chemical compound with the molecular formula C7H5BrN2. It presents as a yellow to brown crystalline solid and is known for its application in the pharmaceutical industry as a key intermediate in the synthesis of various pharmaceutical drugs. 3-Bromo-2-cyano-6-methylpyridine is used in the production of active pharmaceutical ingredients and is also utilized in the manufacturing of agrochemicals. Additionally, 3-Bromo-2-cyano-6-methylpyridine serves as a building block in the creation of various organic chemicals and is commonly employed in research and development laboratories for its versatile chemical properties. Furthermore, it is crucial to handle and store this chemical compound with appropriate safety measures and in a controlled environment to prevent any potential hazards. |
InChI:InChI=1/C7H5BrN2/c1-5-2-3-6(8)7(4-9)10-5/h2-3H,1H3
717843-48-6 Relevant articles
IMIDAZOPYRIMIDINES AS EED INHIBITORS AND THE USE THEREOF
-
Paragraph 0460; 0461, (2021/02/12)
The present disclosure provides compound...
FUSED HETEROCYCLIC DERIVATIVES, THEIR PREPARATION METHODS THEREOF AND MEDICAL USES THEREOF
-
Paragraph 0246; 0427; 0431-0433, (2019/07/03)
The present invention relates to fused h...
ANTIMALARIAL HEXAHYDROPYRIMIDINE ANALOGUES
-
Page/Page column 55, (2019/10/30)
The application relates to a series of 2...
Full synthesis of natural-product (+/-)-cananga odorata alkali and separation method of enantiomers
-
Paragraph 0057; 0070; 0083, (2018/07/07)
The invention discloses full synthesis o...
717843-48-6 Process route
-
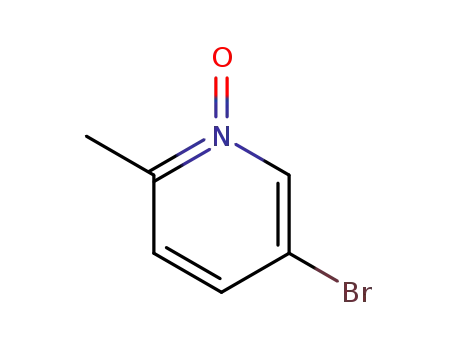
-
31181-64-3
5-bromo-2-methyl-pyridine-N-oxide

-
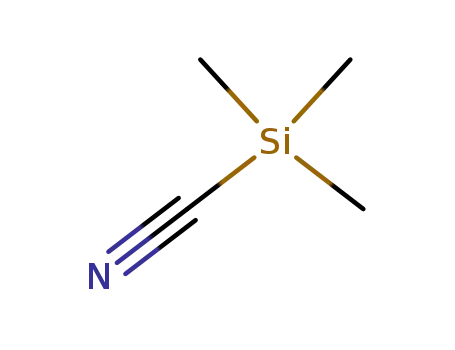
-
7677-24-9
trimethylsilyl cyanide

-
-
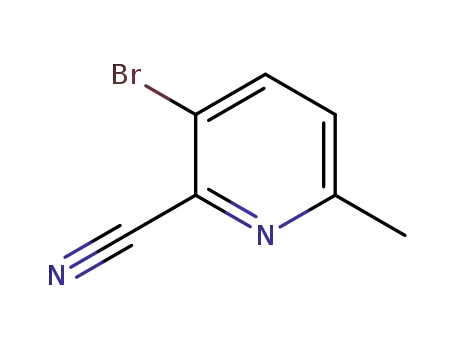
717843-48-6
3-bromo-6-methylpyridine-2-carbonitrile
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
acetonitrile;
for 12h;
Inert atmosphere;
Reflux;
|
85% |
|
With
triethylamine;
In
acetonitrile;
for 12h;
Inert atmosphere;
Reflux;
|
85% |
|
With
triethylamine;
In
acetonitrile;
at 100 ℃;
|
78% |
|
With
triethylamine;
In
acetonitrile;
at 100 ℃;
Inert atmosphere;
|
71% |
|
With
triethylamine;
In
acetonitrile;
at 100 ℃;
|
70% |
|
With
triethylamine;
In
acetonitrile;
at 100 ℃;
|
|
|
With
triethylamine;
In
acetonitrile;
at 100 ℃;
|
-
-
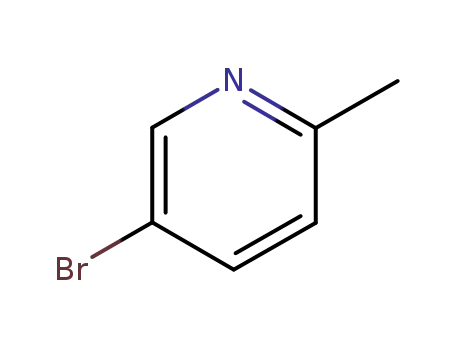
3430-13-5
5-bromo-2-methylpyridine

-
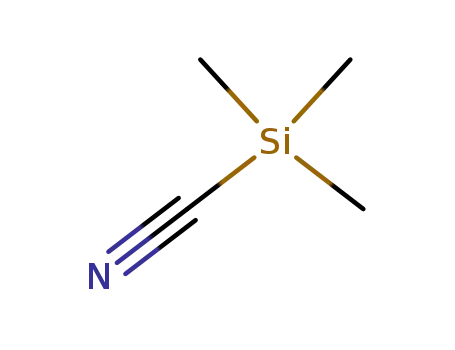
-
7677-24-9
trimethylsilyl cyanide

-

-
717843-48-6
3-bromo-6-methylpyridine-2-carbonitrile
| Conditions | Yield |
|---|---|
|
5-bromo-2-methylpyridine;
With
3-chloro-benzenecarboperoxoic acid;
In
chloroform;
at 20 ℃;
for 4h;
trimethylsilyl cyanide;
With
trimethylamine;
In
acetonitrile;
at 100 ℃;
for 16h;
|
66% |
717843-48-6 Upstream products
-
31181-64-3

5-bromo-2-methyl-pyridine-N-oxide
-
7677-24-9
trimethylsilyl cyanide
-
3430-13-5

5-bromo-2-methylpyridine
717843-48-6 Downstream products
-
1620-75-3
2-Cyano-6-methylpyridine
-
1384199-29-4
6-methyl-3-(2H-1,2,3-triazol-2-yl)picolinonitrile
-
1384199-28-3
6-methyl-3-(1H-1,2,3-triazol-1-yl)-2-pyridinecarbonitrile
-
1252905-76-2
(1R,4S,6R)-4-({[(1,1-dimethylethyl)(diphenyl)silyl]oxy}methyl)-3-{[6-methyl-3-(2-pyrimidinyl)-2-pyridinyl]carbonyl}-3-azabicyclo[4.1.0]heptane
Relevant Products
-
Methyl 6-bromo-3-hydroxy-2-pyridinecarboxylate
CAS:321601-48-3
-
2,6-dioxopyrazine/DAPO
CAS:41536-72-5
-
1,3-Phenylenediacetonitrile
CAS:626-22-2