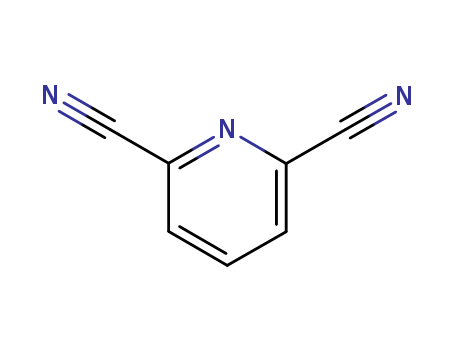
2893-33-6
- Product Name:Pyridine-2,6-dinitrile
- Molecular Formula:C7H3N3
- Purity:99%
- Molecular Weight:129.121
Product Details
pd_meltingpoint:123-127 °C
Purity:99%
Buy reliable Quality Pyridine-2,6-dinitrile 2893-33-6 raw material with Honest Price
- Molecular Formula:C7H3N3
- Molecular Weight:129.121
- Vapor Pressure:0.00213mmHg at 25°C
- Melting Point:123-127 °C
- Refractive Index:1.567
- Boiling Point:289.983 °C at 760 mmHg
- PKA:-5.87±0.10(Predicted)
- Flash Point:107.216 °C
- PSA:60.47000
- Density:1.254 g/cm3
- LogP:0.82496
2,6-Pyridinedicarbonitrile(Cas 2893-33-6) Usage
|
General Description |
2,6-Pyridinedicarbonitrile is a heterocyclic dinitrile. Its biotransformation by Rhodococcus erythropolis A4 to 6-cyanopyridine-2-carboxamide has been reported. |
InChI:InChI=1/C7H3N3/c8-4-6-2-1-3-7(5-9)10-6/h1-3H
2893-33-6 Relevant articles
Multiplying the electron storage capacity of a bis-tetrazine pincer ligand
Benson, Christopher R.,Hui, Alice K.,Parimal, Kumar,Cook, Brian J.,Chen, Chun-Hsing,Lord, Richard L.,Flood, Amar H.,Caulton, Kenneth G.
, p. 6513 - 6524 (2014)
An unexpected doubling in redox storage ...
Syntheses of dual-radioisotope-labeled CP-I, a GABAA receptor partial agonist
Zhang, Yinsheng,Greenfield, Laura,Hong, Yang
, p. 411 - 417 (2011)
CP-I is a potent subtype-selective GABAA...
Experimental and theoretical studies of a triazole ligand and complexes formed with the lanthanides
Drew, Michael G. B.,Hudson, Michael J.,Iveson, Peter B.,Madic, Charles,Russell, Mark L.
, p. 2433 - 2440 (1999)
The crystal structure of 2DBTZP·H2O [DBT...
An example of unusual pyridine donor Schiff base uranyl (UO22+) complexes
Hardy,Wyss,Eddy,Gorden
, p. 5718 - 5720 (2017)
The pentadentate coordination environmen...
Environmentally benign and economic synthesis of covalent triazine-based frameworks
Zhang, Ling,Liu, Xue,Yang, Rui-Xia,Huang, Nian-Yu,Deng, Wei-Qiao
, p. 583 - 588 (2017)
Covalent triazine-based frameworks (CTFs...
Bis-(1,2,4-triazin-3-yl) ligand structure driven selectivity reversal between Am3+and Cm3+: solvent extraction and DFT studies
Ansari, S. A.,Bhattacharyya, Arunasis,Karthikeyan, N. S.,Mohapatra, P. K.,Rao, T. S.,Ravichandran, C.,Seshadri, H.,Venkatachalapathy, B.
, p. 7783 - 7790 (2021/06/16)
Selectivity between Am3+and Cm3+was inve...
Immobilized palladium nanoparticles on a cyclodextrin-polyurethane nanosponge (Pd-CD-PU-NS): An efficient catalyst for cyanation reaction in aqueous media
Khajeh Dangolani, Soheila,Sharifat, Sara,Panahi, Farhad,Khalafi-Nezhad, Ali
supporting information, p. 256 - 265 (2019/06/07)
Immobilized palladium nanoparticles on a...
Synthesis of bis(amidoxime)s and evaluation of their properties as uranyl-complexing agents
Stemper, Jérémy,Tuo, Wei,Mazarío, Eva,Helal, Ahmed S.,Djurovic, Alexandre,Lion, Claude,El Hage Chahine, Jean-Michel,Maurel, Fran?ois,Hémadi, Miryana,Le Gall, Thierry
, p. 2641 - 2649 (2018/04/20)
Uranium pollution involves high toxicity...
2893-33-6 Process route
-
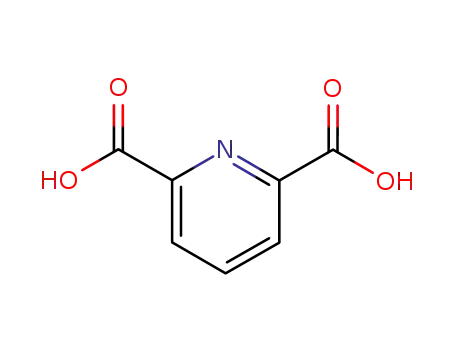
-
499-83-2
Pyridine-2,6-dicarboxylic acid

-
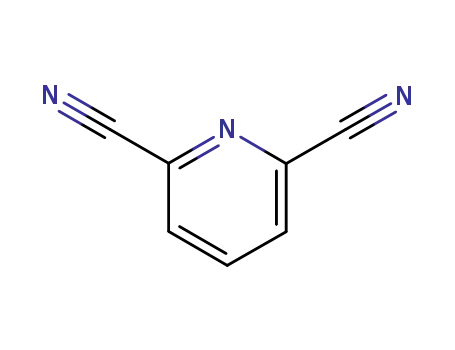
-
2893-33-6
pyridine-2,6-dinitrile
| Conditions | Yield |
|---|---|
|
With
phosphorus pentachloride; ammonia;
In
acetonitrile;
at -40 - 20 ℃;
for 2h;
Temperature;
Solvent;
|
88% |
|
Multi-step reaction with 3 steps
1: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / Reflux
2: ammonia / water; 1,4-dioxane / 0.5 h
3: trichlorophosphate / N,N-dimethyl-formamide / 20 °C
With
thionyl chloride; ammonia; N,N-dimethyl-formamide; trichlorophosphate;
In
1,4-dioxane; dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: sulfuric acid / 30 - 70 °C
2: ammonium hydroxide / 40 °C
3: triethylamine; trifluoroacetic anhydride / tetrahydrofuran / 0 - 25 °C
With
ammonium hydroxide; sulfuric acid; triethylamine; trifluoroacetic anhydride;
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 3 steps
1: thionyl chloride / 14 h / 0 °C / Cooling with ice; Reflux
2: ammonia / water / 1 h / 40 °C
3: trichlorophosphate / N,N-dimethyl-formamide / 0 - 20 °C
With
thionyl chloride; ammonia; trichlorophosphate;
In
water; N,N-dimethyl-formamide;
|
-
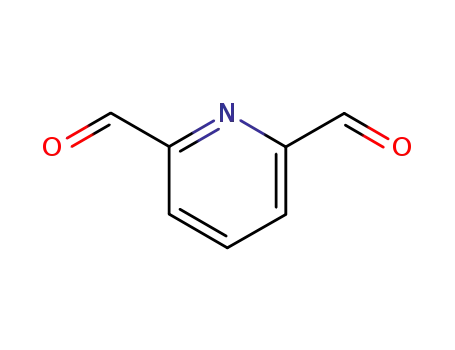
-
5431-44-7
2,6-Pyridinedicarboxaldehyde

-

-
2893-33-6
pyridine-2,6-dinitrile
| Conditions | Yield |
|---|---|
|
With
ammonium acetate; phenyltrimethylammonium tribromide;
In
dichloromethane;
at 20 ℃;
for 16h;
|
91% |
|
Multi-step reaction with 2 steps
1: methanol / 12 h / 20 °C
2: 90 percent / dimethyl sulfate; potassium carbonate / acetonitrile / 16 h / Heating
With
potassium carbonate; dimethyl sulfate;
In
methanol; acetonitrile;
|
2893-33-6 Upstream products
-
4663-97-2
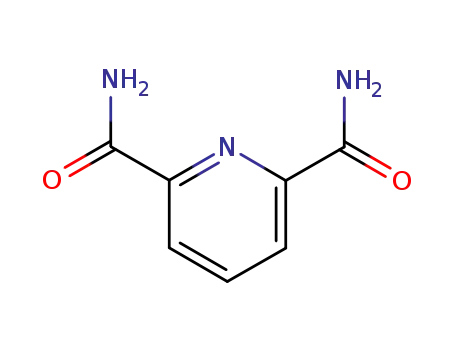
pyridine-2,6-dicarboxylic acid diamide
-
2402-98-4
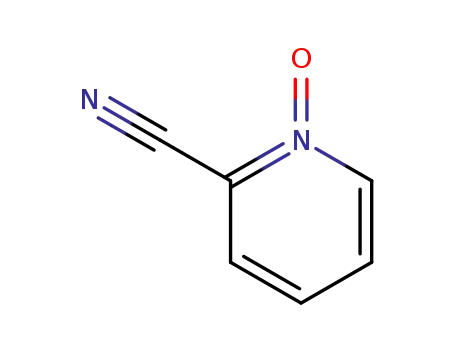
2-cyanopyridine N-oxide
-
7677-24-9
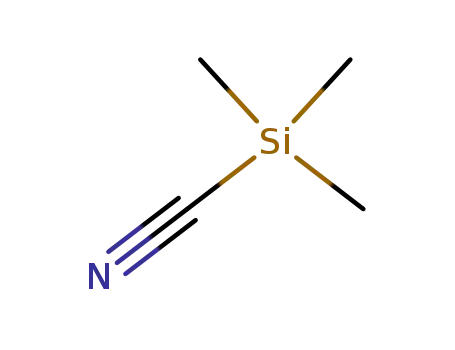
trimethylsilyl cyanide
-
100140-49-6
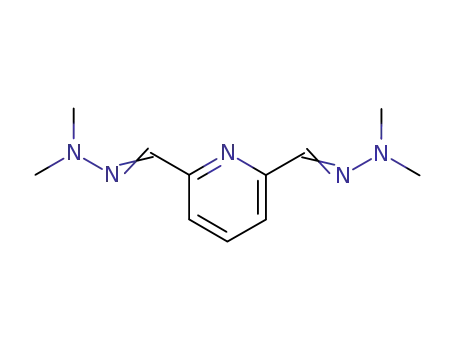
N'-[6-(dimethyl-hydrazonomethyl)-pyridin-2-ylmethylene]-N,N-dimethyl-hydrazine
2893-33-6 Downstream products
-
34984-16-2
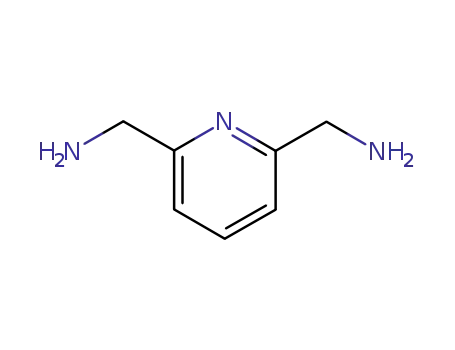
2,6-bis(aminomethyl)pyridine
-
52368-18-0
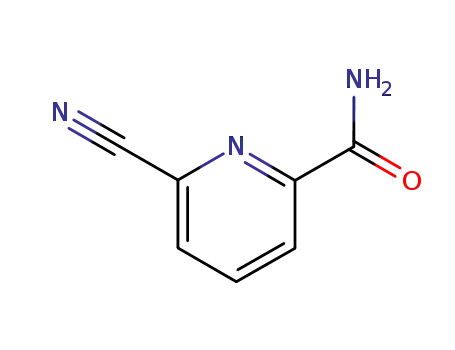
6-Cyan-pyridin-2-carbonsaeure-amid
-
4663-97-2

pyridine-2,6-dicarboxylic acid diamide
-
499-83-2

Pyridine-2,6-dicarboxylic acid
Relevant Products
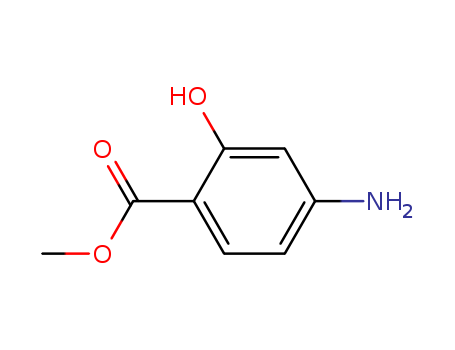
-
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
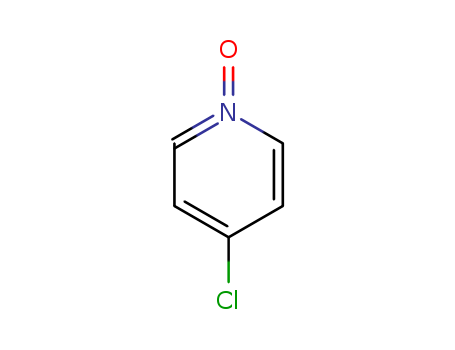
-
4-chloropyridine N-oxide
CAS:1121-76-2
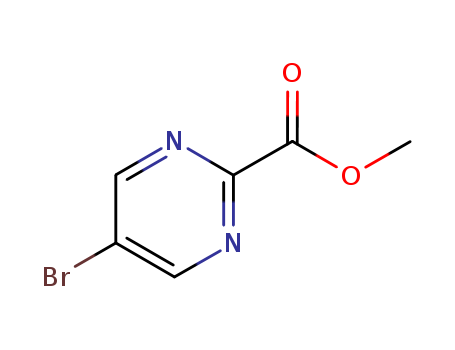
-
Methyl 5-bromo-2-pyrimidinyl formate
CAS:89581-38-4