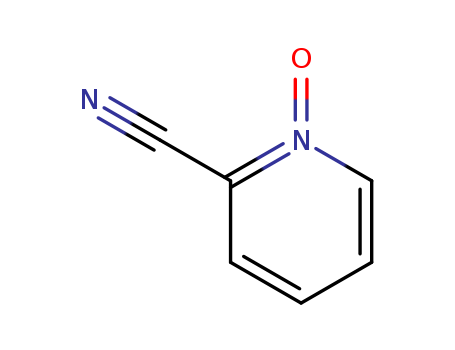
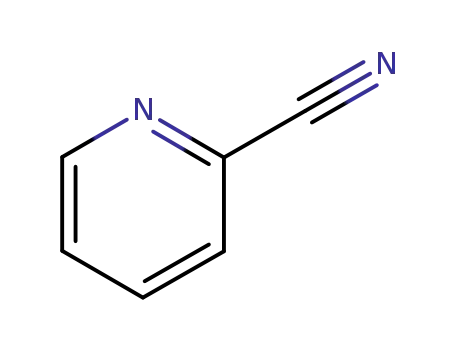
2402-98-4
- Product Name:2-cyanopyridine-N-oxide
- Molecular Formula:C6H4 N2 O
- Purity:99%
- Molecular Weight:120.111
Product Details
pd_meltingpoint:123-124 °C
Purity:99%
Perfect Factory Offer Excellent quality 2-cyanopyridine-N-oxide 2402-98-4 with Safe Shipping
- Molecular Formula:C6H4 N2 O
- Molecular Weight:120.111
- Vapor Pressure:4.63E-06mmHg at 25°C
- Melting Point:123-124 °C
- Boiling Point:382.7°Cat760mmHg
- PKA:-1.92±0.10(Predicted)
- Flash Point:185.3°C
- PSA:49.25000
- Density:1.14g/cm3
- LogP:0.98678
pyridine-2-carbonitrile 1-oxide(Cas 2402-98-4) Usage
|
General Description |
Pyridine-2-carbonitrile 1-oxide is a chemical compound with the molecular formula C6H4N2O. It is a nitrogen-containing heterocyclic compound that contains a pyridine ring and a nitrile group. The 1-oxide functionality indicates the presence of an oxygen atom attached to the first position of the pyridine ring. pyridine-2-carbonitrile 1-oxide is used in various chemical reactions and synthesis processes, and it has potential applications in pharmaceuticals and agrochemicals. It is important to handle pyridine-2-carbonitrile 1-oxide with caution, as it may have hazardous properties and should be used in a controlled and safe manner. |
InChI:InChI=1/C6H4N2O/c7-5-6-3-1-2-4-8(6)9/h1-4H
2402-98-4 Relevant articles
Regioselective Three-Component Reaction of Pyridine N-Oxides, Acyl Chlorides, and Cyclic Ethers
Jones, D. Heulyn,Kay, Steven T.,McLellan, Jayde A.,Kennedy, Alan R.,Tomkinson, Nicholas C. O.
supporting information, p. 3512 - 3515 (2017/07/17)
A novel three-component reaction of pyri...
Catalyst-free and selective oxidation of pyridine derivatives and tertiary amines to corresponding N-oxides with 1,2-diphenyl-1,1,2,2-tetrahydroperoxyethane
Azarifar, Davood,Mahmoudi, Boshra
, p. 645 - 651 (2016/02/19)
The catalyst-free oxidation of various p...
Solvent- and halide-free synthesis of pyridine-2-yl substituted ureas through facile C-H functionalization of pyridine: N -oxides
Rassadin, Valentin A.,Zimin, Dmitry P.,Raskil'dina, Gulnara Z.,Ivanov, Alexander Yu.,Boyarskiy, Vadim P.,Zlotskii, Semen S.,Kukushkin, Vadim Yu.
supporting information, p. 6630 - 6636 (2018/03/01)
A novel solvent- and halide-free atom-ec...
Base free regioselective synthesis of α-triazolylazine derivatives
Harisha, Mysore Bhyrappa,Nagaraj, Muthupandi,Muthusubramanian, Shanmugam,Bhuvanesh, Nattamai
, p. 58118 - 58124 (2016/07/06)
A regioselective α-heteroarylation follo...
2402-98-4 Process route
-
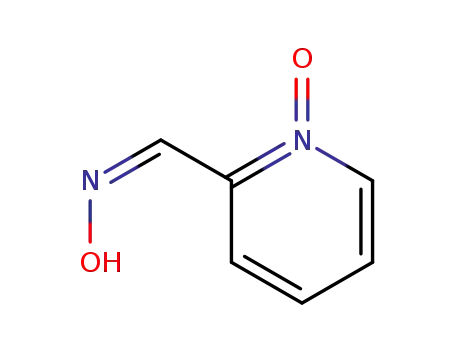
-
24145-26-4
(Z)-2-pyridinecarbaldehyde 1-oxide oxime

-
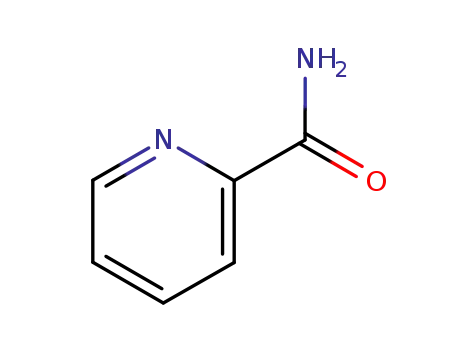
-
1452-77-3
pyridine-2-carboxylic acid amide

-
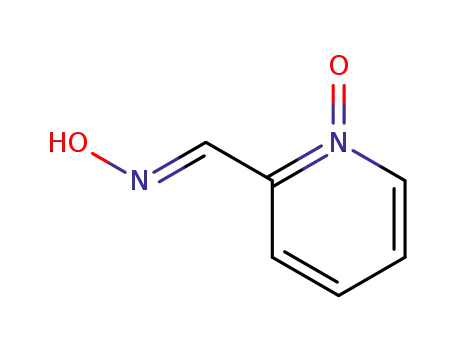
-
24145-28-6
(E)-2-pyridinecarbaldehyde 1-oxide oxime

-
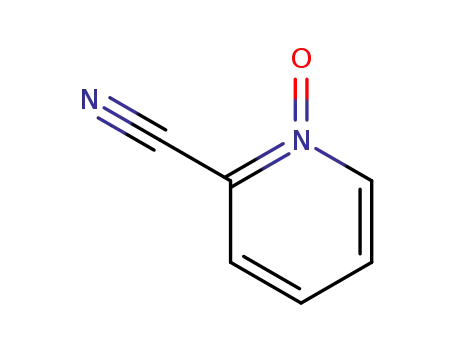
-
2402-98-4
2-cyanopyridine N-oxide

-
-
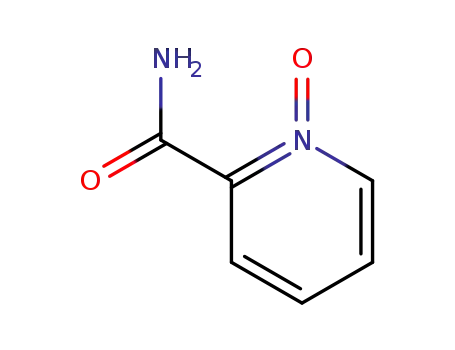
6974-72-7
2-pyridinecarboxamide 1-oxide
| Conditions | Yield |
|---|---|
|
With
C5H11OH; ammonium chloride; sodium amide; isopentyl nitrite;
In
ammonia;
Ambient temperature;
|
40% 14% 12% 5% |
-
-
100-70-9,1232006-36-8
2-Cyanopyridine

-

-
2402-98-4
2-cyanopyridine N-oxide
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide; methyltrioxorhenium(VII);
In
dichloromethane; water;
at 20 ℃;
Inert atmosphere;
|
100% |
|
With
HOF* CH3CN;
In
chloroform;
at 0 - 20 ℃;
|
98% |
|
With
dihydrogen peroxide; methyltrioxorhenium(VII);
In
dichloromethane; water;
for 24h;
Ambient temperature;
|
94% |
|
With
1,2-diphenyl-1,1,2,2-tetrahydroperoxyethane;
In
acetonitrile;
at 20 ℃;
for 0.0833333h;
|
93% |
|
With
bis-trimethylsilanyl peroxide; per-rhenic acid;
In
dichloromethane; water;
at 24 ℃;
for 15h;
|
92% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
for 24h;
Inert atmosphere;
|
88% |
|
With
titanium silicate; dihydrogen peroxide;
In
methanol;
at 60 ℃;
for 25h;
|
81.3% |
|
With
oxygen; isobutyraldehyde;
In
1,2-dichloro-ethane;
at 30 ℃;
for 24h;
|
76% |
|
With
oxygen;
ruthenium trichloride;
In
1,2-dichloro-ethane;
at 20 ℃;
for 20h;
under 760 Torr;
|
75% |
|
With
dihydrogen peroxide; acetic anhydride;
In
water;
at 80 ℃;
for 12h;
|
62% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 20 ℃;
|
56% |
|
With
dihydrogen peroxide; acetic acid;
|
|
|
2-Cyanopyridine;
With
dihydrogen peroxide;
methyltrioxorhenium(VII);
In
dichloromethane; water;
at 20 ℃;
for 6h;
manganese(IV) oxide;
In
dichloromethane; water;
at 20 ℃;
for 1h;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
|
2402-98-4 Upstream products
-
100-70-9

2-Cyanopyridine
-
6974-72-7

2-pyridinecarboxamide 1-oxide
-
24145-26-4

(Z)-2-pyridinecarbaldehyde 1-oxide oxime
-
1452-77-3

pyridine-2-carboxylic acid amide
2402-98-4 Downstream products
-
100-70-9

2-Cyanopyridine
-
1192-31-0
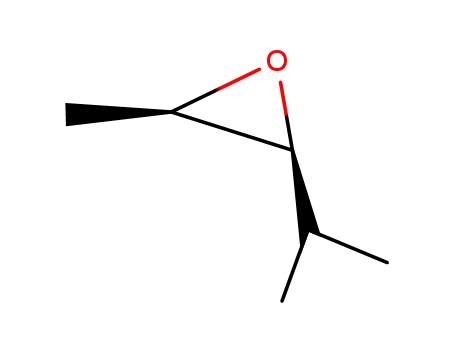
cis-2,3-epoxy-4-methylpentane
-
1192-31-0
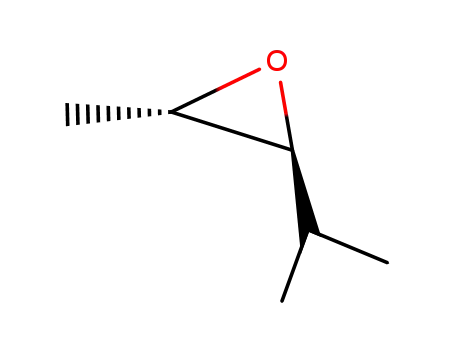
trans-2,3-epoxy-4-methylpentane
-
72221-03-5
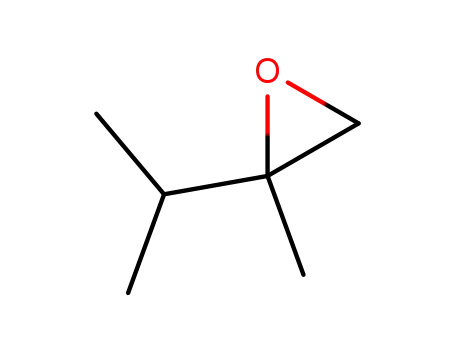
2,3-dimethyl-1,2-epoxybutane
Relevant Products
-
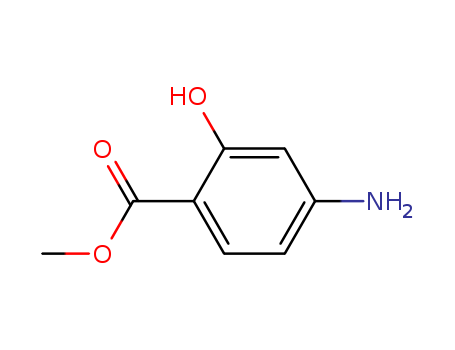
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
-
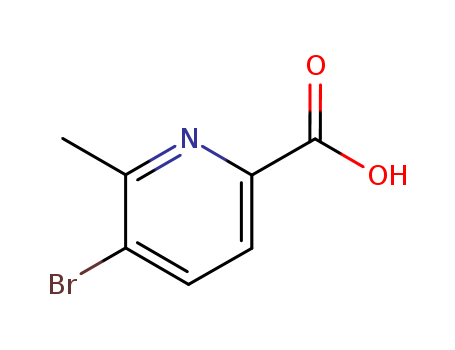
3-bromo-2-methylpyridine-6-carboxylic acid
CAS:137778-20-2
-
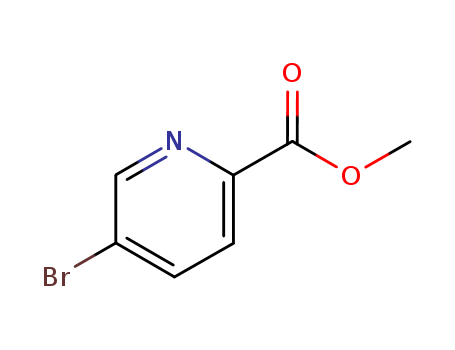
5-bromopyridine-2-carboxylic acid methyl ester
CAS:29682-15-3