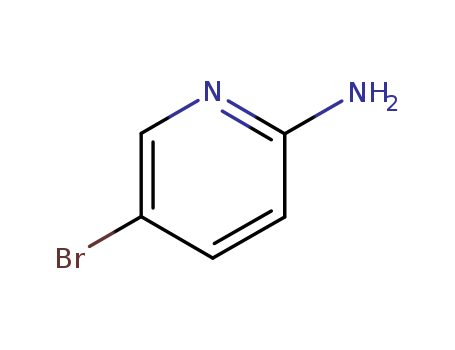
1072-97-5
- Product Name:2-Amino-5-bromopyridine
- Molecular Formula:C5H5BrN2
- Purity:99%
- Molecular Weight:173.012
Product Details
pd_meltingpoint:135-138 °C
Appearance:Light yellow crystal
Purity:99%
Perfect Factory Offer Excellent quality 2-Amino-5-bromopyridine 1072-97-5 with Safe Shipping
- Molecular Formula:C5H5BrN2
- Molecular Weight:173.012
- Appearance/Colour:Light yellow crystal
- Vapor Pressure:0.0643mmHg at 25°C
- Melting Point:135-138 °C
- Refractive Index:1.5182 (estimate)
- Boiling Point:230.9 °C at 760 mmHg
- PKA:4.65±0.13(Predicted)
- Flash Point:93.4 °C
- PSA:38.91000
- Density:1.71 g/cm3
- LogP:2.00750
2-Amino-5-bromopyridine(Cas 1072-97-5) Usage
|
General Description |
2-Amino-5-bromopyridine is a brominated aromatic amine reagent and is used for labeling of model reducing-end oligosaccharides via reductive amination. |
InChI:InChI=1/C5H5BrN2/c6-4-1-2-5(7)8-3-4/h1-3H,(H2,7,8)/p+1
1072-97-5 Relevant articles
Pyridinium-N-(2-pyridyl)aminides: A selective approach to substituted 2-aminopyridines
Carceller, Rosa,Garcia-Navio, Jose L.,Izquierdo, Maria L.,Alvarez-Builla, Julio
, p. 2019 - 2020 (1993)
Differently substituted 2-aminopyridines...
Tertiary formylated amines by microwave irradiation of N,N-dimethyl- N′-(2-pyridyl) formamidines with methyl vinyl ketone
Gomez-Garcia, Omar,Salgado-Zamora, Hector,Campos-Aldrete, Elena
, p. 21 - 23 (2014)
Treatment of N,N-dimethyl-N′-(2-pyridyl)...
-
Bradlow,Vanderwerf
, p. 73,79 (1951)
-
Preparation of 2,3-Disubstituted 5-Bromo-1 H -pyrrolo[2,3- b ]pyridine Framework by Fischer Cyclization
Alekseyev, Roman S.,Amirova, Sabina R.,Terenin, Vladimir I.
, p. 3169 - 3178 (2015)
A simple synthesis of some hard-to-reach...
Metal-Free Reduction of Aromatic Nitro Compounds to Aromatic Amines with B2pin2 in Isopropanol
Lu, Hongtao,Geng, Zhiyue,Li, Jingya,Zou, Dapeng,Wu, Yusheng,Wu, Yangjie
, p. 2774 - 2776 (2016)
A metal-free reduction of aromatic nitro...
Nitrogen-doped graphene-activated iron-oxide-based nanocatalysts for selective transfer hydrogenation of nitroarenes
Jagadeesh, Rajenahally V.,Natte, Kishore,Junge, Henrik,Beller, Matthias
, p. 1526 - 1529 (2015)
Nanoscaled iron oxides on carbon were mo...
The acid-catalysed synthesis of 7-azaindoles from 3-alkynyl-2- aminopyridines and their antimicrobial activity
Leboho, Tlabo C.,Van Vuuren, Sandy F.,Michael, Joseph P.,De Koning, Charles B.
, p. 307 - 315 (2014)
The synthesis of 7-azaindoles from 3-alk...
Handling Hydrogen Peroxide Oxidations on a Large Scale: Synthesis of 5-Bromo-2-nitropyridine
Agosti, Alessandro,Bertolini, Giorgio,Bruno, Giacomo,Lautz, Christian,Glarner, Thomas,Deichtmann, Walter
, p. 451 - 459 (2017)
5-Bromo-2-nitropyridine was prepared fro...
Directing Group Enables Electrochemical Selectively Meta-Bromination of Pyridines under Mild Conditions
Wu, Yanwei,Xu, Shanghui,Wang, Hong,Shao, Dongxu,Qi, Qiqi,Lu, Yi,Ma, Li,Zhou, Jianhua,Hu, Wei,Gao, Wei,Chen, Jianbin
, p. 16144 - 16150 (2021)
Without the use of catalysts and oxidant...
Halogenation of pyridinium-N-(2'-pyridyl)aminide: An easy synthesis of halo-2-aminopyridines
Burgos,Delgado,Garcia-Navio,Izquierdo,Alvarez-Builla
, p. 8649 - 8654 (1995)
The regioselective halogenation of pyrid...
Synthesis of a fluorine-18 labeled derivative of epibatidine for in vivo nicotinic acetylcholine receptor PET imaging
Dolci, Lilian,Dolle, Frederic,Valette, Heric,Vaufrey, Francoise,Fuseau, Chantal,Bottlaender, Michel,Crouzel, Christian
, p. 467 - 479 (1999)
Epibatidine (exo-2-(2'-chloro-5'-pyridyl...
Mild regioselective halogenation of activated pyridines with N-bromosuccinimide
Canibano,Rodriguez,Santos,Sanz-Tejedor,Carreno,Gonzalez,Garcia-Ruano
, p. 2175 - 2179 (2001)
Regioselective mono and dihalogenations ...
-
Sasaki et al.
, p. 5121,5129 (1971)
-
Electrochemical C-H Halogenations of Enaminones and Electron-Rich Arenes with Sodium Halide (NaX) as Halogen Source for the Synthesis of 3-Halochromones and Haloarenes
Jin, Jun,Lin, Yan,Liu, Yunyun,Wan, Jie-Ping,Wang, Chaoli
, p. 12378 - 12385 (2021/09/07)
Without employing an external oxidant, t...
Method for synthesizing 2-amino-3-chloro-5-trifluoromethylpyridine
-
Paragraph 0007; 0020-0021; 0026-0027; 0032-0033, (2021/02/06)
The invention discloses a method for syn...
Synthetic method of medicinal raw material 2 and 5 - dibromopyridine
-
Paragraph 0018; 0021; 0024, (2021/10/05)
The invention relates to the technical f...
1072-97-5 Process route
-
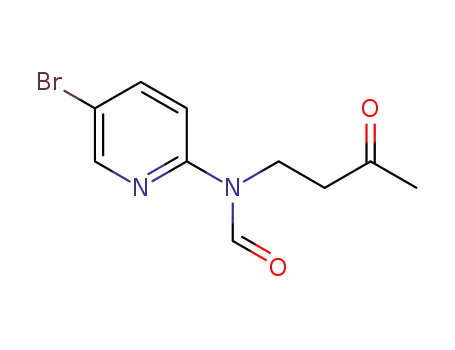
-
5-bromo-N-formyl-N-(3-oxobutyl)-2-pyridylamine

-
-
1072-97-5
5-bromo-2-pyridylamine

-
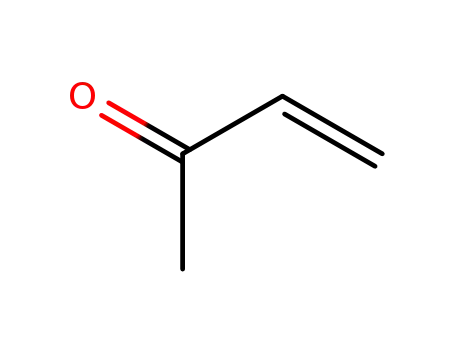
-
78-94-4,25038-87-3
methyl vinyl ketone
| Conditions | Yield |
|---|---|
|
With
ammonium acetate;
|
-
-
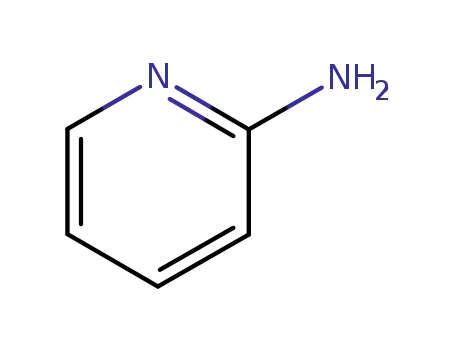
504-29-0
2-aminopyridine

-

-
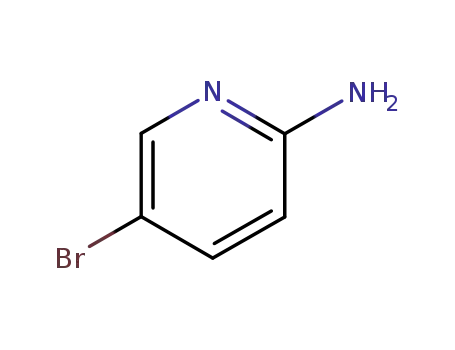
1072-97-5
5-bromo-2-pyridylamine

-
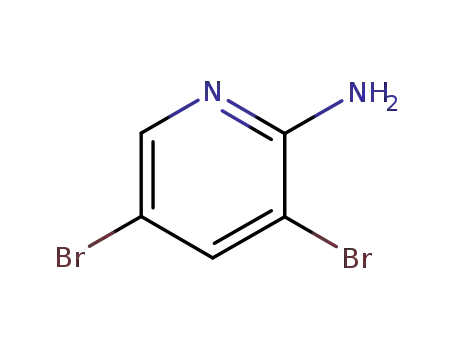
-
35486-42-1
2-amino-3,5-dibromopyridine
| Conditions | Yield |
|---|---|
|
With
tetra-N-butylammonium tribromide;
In
chloroform;
at 20 ℃;
for 0.0333333h;
other reagent: tetraphenylphosphonium tribromide;
|
2% 98% |
|
With
N-Bromosuccinimide; lithium perchlorate; silica gel;
In
dichloromethane;
at 20 ℃;
for 0.0833333h;
|
90% 10% |
|
With
bromine;
In
ethanol;
at 20 ℃;
|
76% 12% |
|
With
bromine; sodium hydrogencarbonate;
In
dichloromethane; acetonitrile;
1.) 2 h, 2.) 1 h;
|
23% 68% |
|
2-aminopyridine;
With
bromine; acetic acid;
at 20 - 50 ℃;
With
sodium hydroxide;
|
67% |
|
With
bromine; sodium hydrogencarbonate;
In
dichloromethane; acetonitrile;
for 1h;
|
46% 10% |
|
With
bromine;
In
ethanol;
at 20 ℃;
for 2h;
|
44% |
|
With
ethanol; bromine;
|
|
|
With
sulfuric acid; bromine;
|
|
|
With
N-Bromosuccinimide;
In
acetonitrile;
at -8 - -5 ℃;
Temperature;
Thermodynamic data;
|
15 %Chromat. |
1072-97-5 Upstream products
-
504-29-0

2-aminopyridine
-
39856-50-3
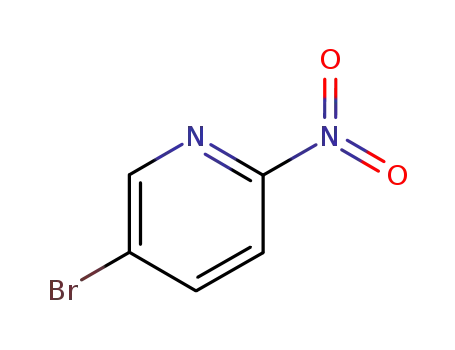
5-bromo2-nitropyridine
-
157020-26-3
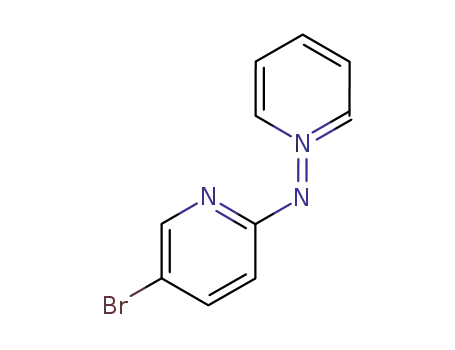
pyridinium N-<2'-(5'-bromopyridyl)>aminide
-
33245-29-3
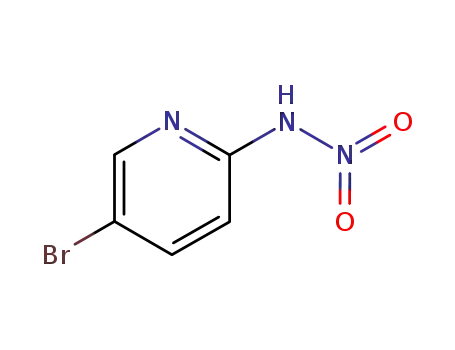
5-bromo-2-nitraminopyridine
1072-97-5 Downstream products
-
1086-87-9
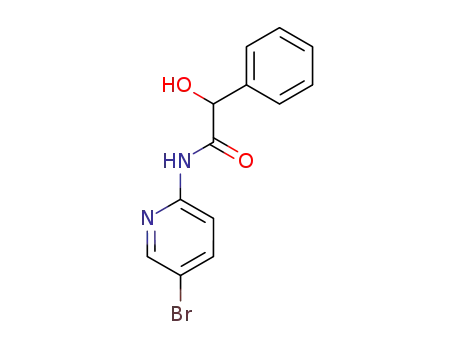
N-(5-bromo-[2]pyridyl)-mandelamide
-
856850-36-7
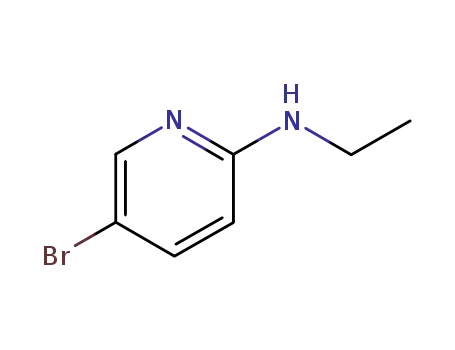
5-bromo-N-ethylpyridin-2-amine
-
857970-06-0
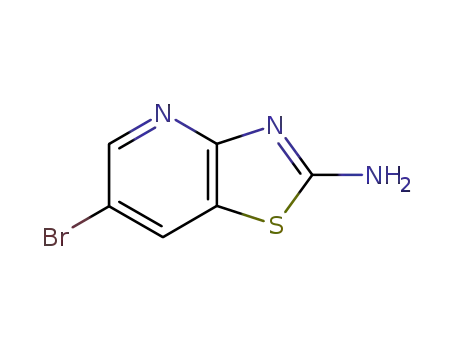
2-amino-6-bromo[1,3]thiazolo[4,5-b]pyridine
-
64500-11-4
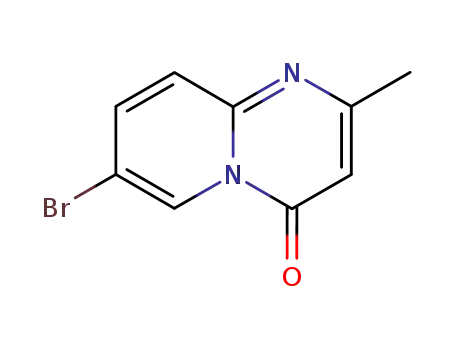
7- bromo-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
Relevant Products
-
3-bromo-6-methylpyridine-2-carboxylic acid
CAS:717843-48-6
-
2,6-difluoronitrobenzene
CAS:19064-24-5
-
Niacin oxide
CAS:2398-81-4