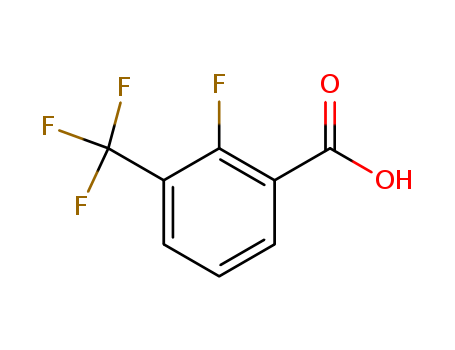
115029-22-6
- Product Name:2-Fluoro-3- (trifluoromethyl) benzoic acid
- Molecular Formula:C8H4F4O2
- Purity:99%
- Molecular Weight:208.112
Product Details
pd_meltingpoint:126-128 °C(lit.)
Appearance:White to beige crystalline powder
Purity:99%
Factory supply 2-Fluoro-3- (trifluoromethyl) benzoic acid 115029-22-6 with sufficient production capacity
- Molecular Formula:C8H4F4O2
- Molecular Weight:208.112
- Appearance/Colour:White to beige crystalline powder
- Vapor Pressure:0.012mmHg at 25°C
- Melting Point:126-128 °C(lit.)
- Boiling Point:249.6 °C at 760 mmHg
- Flash Point:104.7 °C
- PSA:37.30000
- Density:1.489 g/cm3
- LogP:2.54270
2-FLUORO-3-(TRIFLUOROMETHYL)BENZOIC ACID(Cas 115029-22-6) Usage
InChI:InChI=1/C8H4F4O2/c9-6-4(7(13)14)2-1-3-5(6)8(10,11)12/h1-3H,(H,13,14)/p-1
115029-22-6 Relevant articles
Discovery of Orally Bioavailable Ligand Efficient Quinazolindiones as Potent and Selective Tankyrases Inhibitors
Qin, Donghui,Lin, Xiaojuan,Liu, Zhi,Chen, Yan,Zhang, Zhiliu,Wu, Chengde,Liu, Linlin,Pan, Yan,Laquerre, Sylvie,Emery, John,Fergusson, Jeff,Roland, Kimberly,Keenan, Rick,Oliff, Allen,Kumar, Sanjay,Cheung, Mui,Su, Dai-Shi
, p. 1005 - 1010 (2021)
We report herein the discovery of quinaz...
2-chloro-3-fluoro-4-(trifluoromethyl) benzaldehyde and synthesis method thereof
-
Paragraph 0018-0019; 0030-0031, (2021/04/10)
The invention belongs to the technical f...
P2X7 MODULATORS
-
Paragraph 0284; 0286, (2014/09/29)
The present invention is directed to com...
QUINAZOLINEDIONES AS TANKYRASE INHIBITORS
-
Page/Page column 39, (2014/01/07)
This invention relates to the use of qui...
115029-22-6 Process route
-
-
124-38-9,18923-20-1
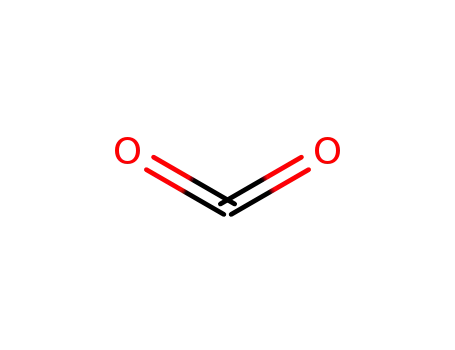
carbon dioxide

-
-
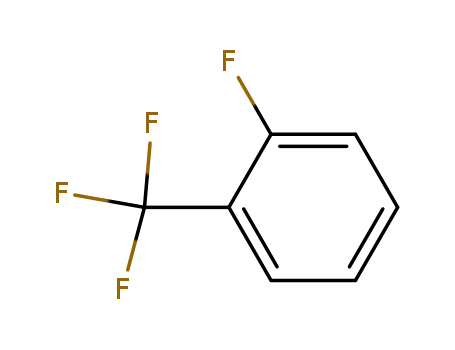
392-85-8
1-fluoro-2-trifluoromethylbenzene

-
-
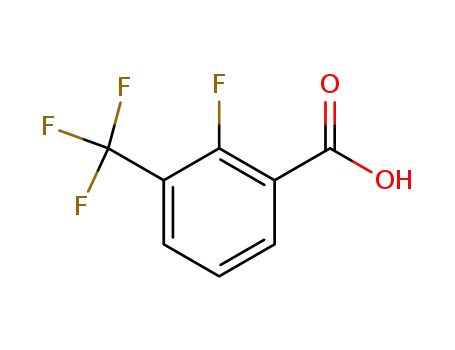
115029-22-6
2-fluoro-3-(trifluoromethyl)benzoic acid
| Conditions | Yield |
|---|---|
|
1-fluoro-2-trifluoromethylbenzene;
With
lithium diisopropyl amide;
In
tetrahydrofuran;
at -80 ℃;
for 0.666667h;
Inert atmosphere;
carbon dioxide;
In
tetrahydrofuran;
for 2h;
Inert atmosphere;
With
hydrogenchloride;
In
water; ethyl acetate;
Temperature;
|
90.4% |
|
1-fluoro-2-trifluoromethylbenzene;
With
lithium diisopropyl amide;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
carbon dioxide;
In
tetrahydrofuran;
at -78 ℃;
for 1.83333h;
|
84% |
|
1-fluoro-2-trifluoromethylbenzene;
With
lithium diisopropyl amide;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
carbon dioxide;
In
tetrahydrofuran;
at -78 ℃;
for 1.83333h;
Inert atmosphere;
|
84% |

-

-
115029-22-6
2-fluoro-3-(trifluoromethyl)benzoic acid
| Conditions | Yield |
|---|---|
|
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
for 4h;
|
115029-22-6 Upstream products
-
124-38-9

carbon dioxide
-
392-85-8

1-fluoro-2-trifluoromethylbenzene
115029-22-6 Downstream products
-
680610-54-2
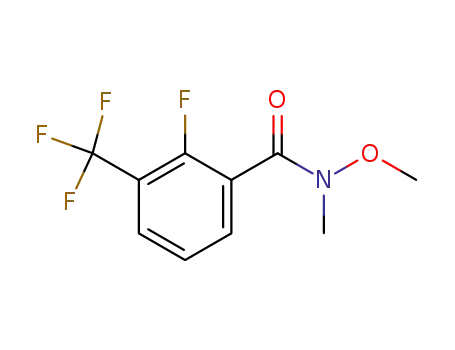
2-fluoro-N-methoxy-N-methyl-3-(trifluoromethyl)benzamide
-
178748-05-5
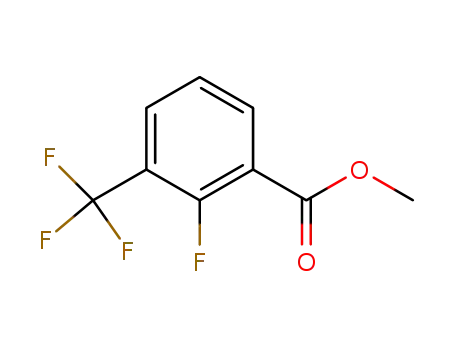
methyl 2-fluoro-3-(trifluoromethyl)benzoate
-
164341-19-9
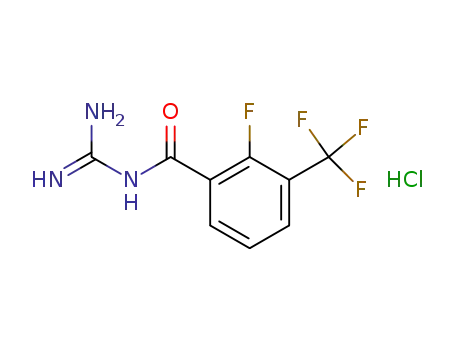
2-Fluoro-3-trifluoromethylbenzoylguanidine hydrochloride
-
885328-05-2
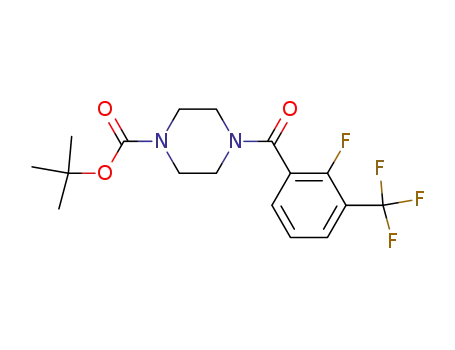
tert-butyl 4-(2-fluoro-3-(trifluoromethyl)benzoyl)piperazine-1-carboxylate
Relevant Products
-
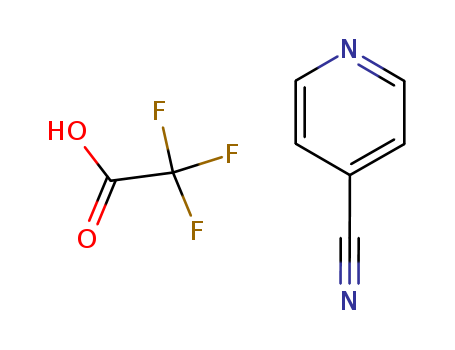
4-cyanopyridine monotrifluoroacetate
CAS:29885-70-9
-
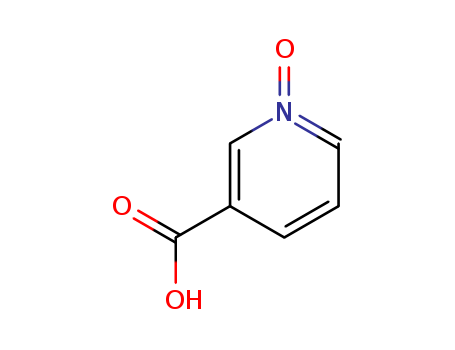
Niacin oxide
CAS:2398-81-4
-
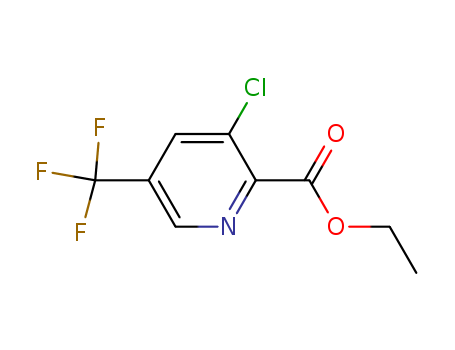
Ethyl 3-chloro-5-trifluoromethylpyridine-2-carboxylate
CAS:128073-16-5