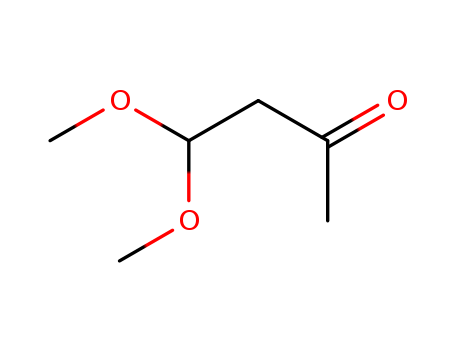
5436-21-5
- Product Name:4,4-dimethoxy-2-butanone
- Molecular Formula:C6H12O3
- Purity:99%
- Molecular Weight:132.159
Product Details
pd_meltingpoint:-82 °C
Appearance:Clear colorless to yellow liquid
Purity:99%
Reliable factory customized supply 4,4-dimethoxy-2-butanone 5436-21-5
- Molecular Formula:C6H12O3
- Molecular Weight:132.159
- Appearance/Colour:Clear colorless to yellow liquid
- Vapor Pressure:1.35mmHg at 25°C
- Melting Point:-82 °C
- Refractive Index:n20/D 1.414(lit.)
- Boiling Point:172.1 °C at 760 mmHg
- Flash Point:49.4 °C
- PSA:35.53000
- Density:0.959 g/cm3
- LogP:0.58440
Acetylacetaldehyde dimethyl acetal(Cas 5436-21-5) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 41, p. 3765, 1976 DOI: 10.1021/jo00885a028Tetrahedron Letters, 28, p. 6657, 1987 DOI: 10.1016/S0040-4039(00)96938-7 |
|
General Description |
4,4-Dimethoxy-2-butanone (Acetylacetaldehyde dimethylacetal) is a ketone. |
InChI:InChI=1/C6H12O3/c1-5(7)4-6(8-2)9-3/h6H,4H2,1-3H3
5436-21-5 Relevant articles
Biosynthesis of polyketide-terpenoid (Meroterpenoid) metabolites andibenin B and andilesin A in aspergillus variecolor
Simpson, Thomas J.,Anmed, Salman A.,McIntyre, C. Rupert,Scott, Fiona E.,Sadler, Ian H.
, p. 4013 - 4034 (1997)
Incorporation of 13C-labelled acetates a...
Synthesis method of 4,4-dimethoxy-2-butanone
-
Paragraph 0017-0037, (2021/11/06)
The invention discloses a synthesis meth...
Electronic Asymmetry of an Annelated Pyridyl-Mesoionic Carbene Scaffold: Application in Pd(II)-Catalyzed Wacker-Type Oxidation of Olefins
Bera, Jitendra K.,Dutta, Indranil,Kunnikuruvan, Sooraj,Reshi, Noor U Din,Saha, Sayantani,Yadav, Suman
, p. 11385 - 11393 (2020/11/23)
The two donor modules of an annelated py...
CuII-Catalyzed Oxidative Formation of 5-Alkynyltriazoles
Liu, Peiye,Brassard, Christopher J.,Lee, Justin P.,Zhu, Lei
supporting information, p. 380 - 390 (2020/01/24)
In an alcoholic solvent under the cataly...
Tropylium salts as efficient organic Lewis acid catalysts for acetalization and transacetalization reactions in batch and flow
Lyons,Crocker,Enders,Nguyen
supporting information, p. 3993 - 3996 (2017/09/08)
Acetalization reactions play significant...
5436-21-5 Process route
-
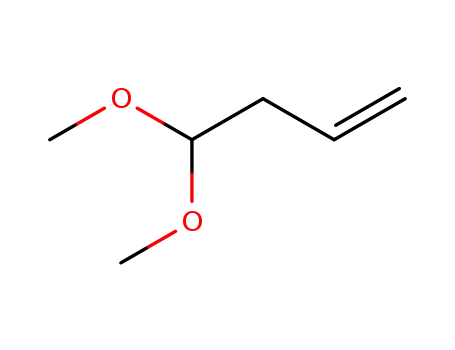
-
72380-56-4
3-butenal dimethyl acetal

-
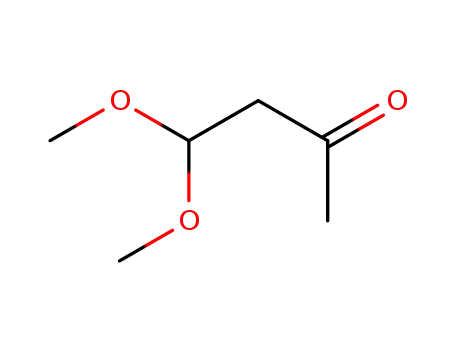
-
5436-21-5
acetylacetaldehyde dimethyl acetal
| Conditions | Yield |
|---|---|
|
With
tert.-butylhydroperoxide; C21H19N5Pd(2+)*2BF4(1-);
In
decane; acetonitrile;
at 70 ℃;
for 24h;
Temperature;
|
95% |
-
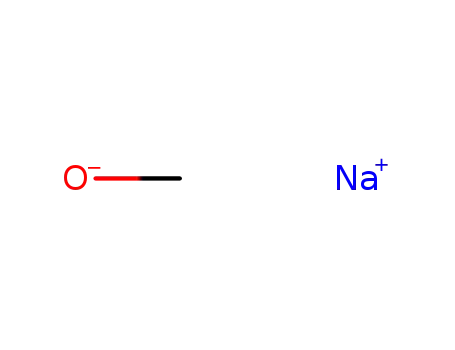
-
124-41-4
sodium methylate

-
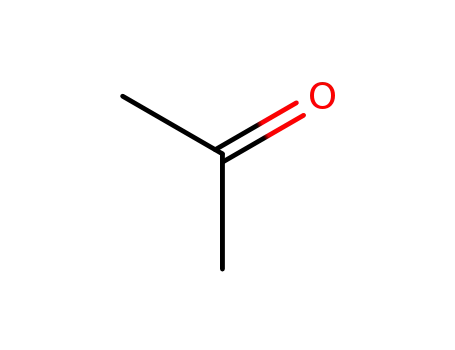
-
67-64-1
acetone

-
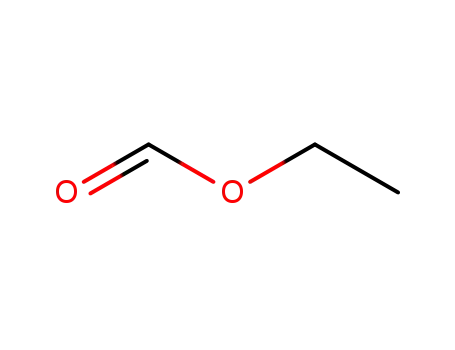
-
109-94-4
formic acid ethyl ester

-

-
5436-21-5
acetylacetaldehyde dimethyl acetal
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
at 0 - 50 ℃;
for 2h;
Temperature;
|
73.2% |
5436-21-5 Upstream products
-
67-56-1
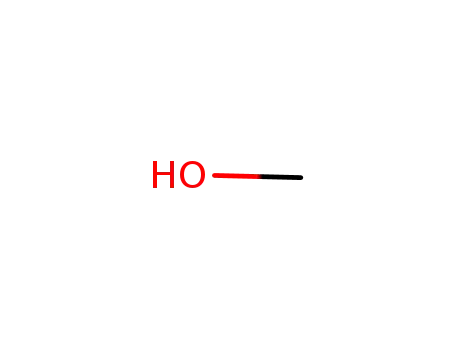
methanol
-
2798-73-4
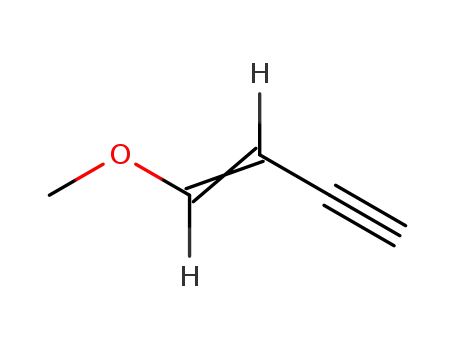
1-methoxy-buten-3-yne
-
56-23-5
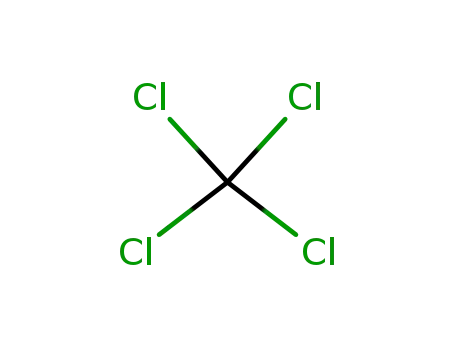
tetrachloromethane
-
7119-27-9
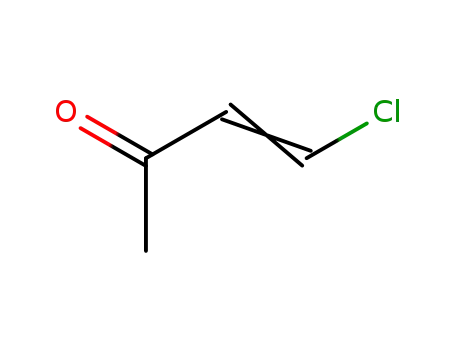
4-chloro-3-buten-2-one
5436-21-5 Downstream products
-
18591-78-1
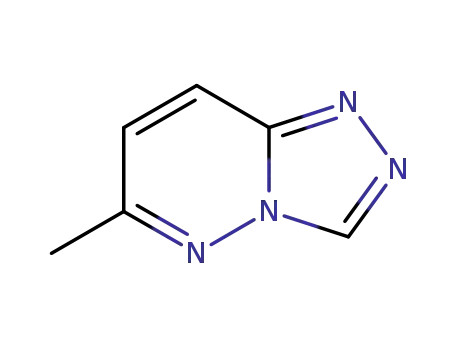
6-Methyl-s-triazolo<4,3-b>pyridazin
-
99969-13-8
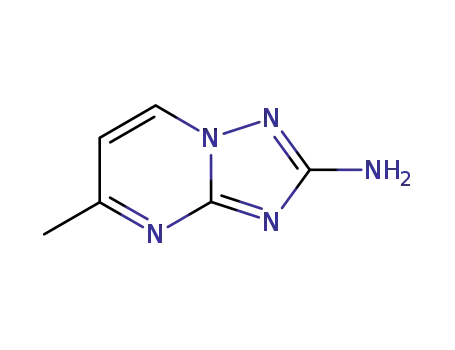
5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-ylamine
-
36357-38-7
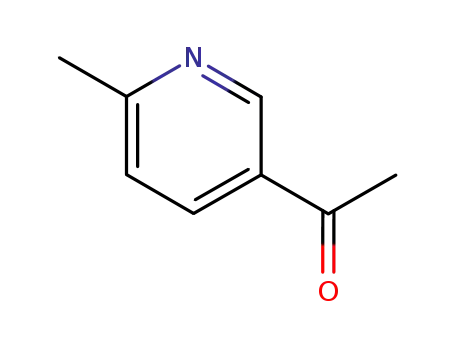
1-(6-methyl-pyridin-3-yl)-ethanone
-
127-73-1
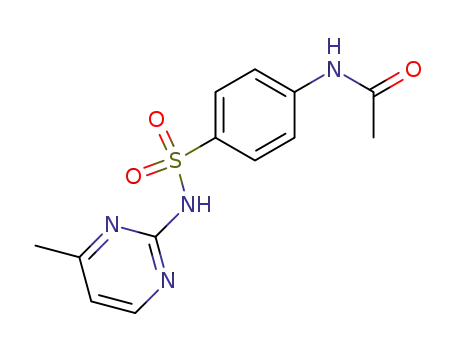
N-[4-(4-Methyl-pyrimidin-2-ylsulfamoyl)-phenyl]-acetamide
Relevant Products
-
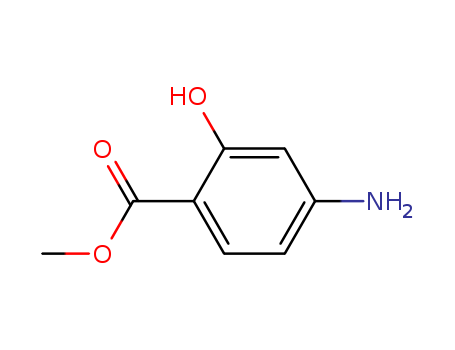
Methyl ortho hydroxy para aminobenzoate
CAS:4136-97-4
-
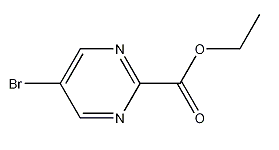
5-bromopyrimidine-2-carboxylic acid ethyl ester
CAS:1197193-30-8