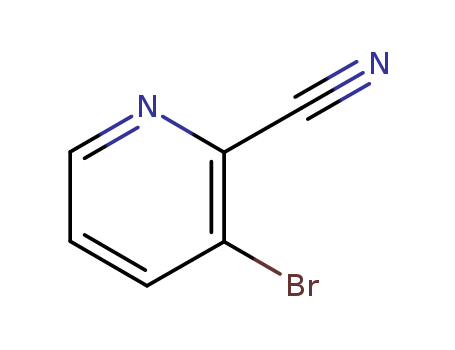
55758-02-6
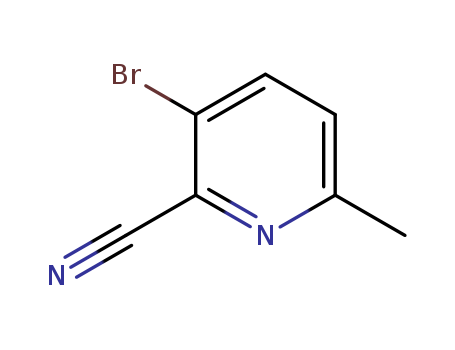
- Product Name:2-Cyano-3-bromopyridine
- Molecular Formula:C6H3BrN2
- Purity:99%
- Molecular Weight:183.007
Product Details
pd_meltingpoint:98.2-99.0 °C
Appearance:slight yellow powder
Purity:99%
Factory Sells Best Quality 2-Cyano-3-bromopyridine 55758-02-6 with stock
- Molecular Formula:C6H3BrN2
- Molecular Weight:183.007
- Appearance/Colour:slight yellow powder
- Vapor Pressure:0.00153mmHg at 25°C
- Melting Point:98.2-99.0 °C
- Refractive Index:1.611
- Boiling Point:295.4 °C at 760 mmHg
- PKA:-2.68±0.10(Predicted)
- Flash Point:132.4 °C
- PSA:36.68000
- Density:1.72 g/cm3
- LogP:1.71578
3-Bromo-2-cyanopyridine(Cas 55758-02-6) Usage
InChI:InChI=1/C6H3BrN2/c7-5-2-1-3-9-6(5)4-8/h1-3H
55758-02-6 Relevant articles
C?H Cyanation of 6-Ring N-Containing Heteroaromatics
Elbert, Bryony L.,Farley, Alistair J. M.,Gorman, Timothy W.,Johnson, Tarn C.,Genicot, Christophe,Lallemand, Bénédicte,Pasau, Patrick,Flasz, Jakub,Castro, José L.,MacCoss, Malcolm,Paton, Robert S.,Schofield, Christopher J.,Smith, Martin D.,Willis, Michael C.,Dixon, Darren J.
supporting information, p. 14733 - 14737 (2017/10/07)
Heteroaromatic nitriles are important co...
CARBACEPHEM β-LACTAM ANTIBIOTICS
-
Page/Page column 77, (2010/04/06)
Carbacephem -lactam antibiotics having s...
CARBACEPHEM β-LACTAM ANTIBIOTICS
-
Page/Page column 90-91, (2009/05/30)
Carbacephem β-lactam antibiotics having ...
Aluminum-catalyzed asymmetric alkylations of pyridyl-substituted alkynyl ketones with dialkylzinc reagents
Friel, Donna K.,Snapper, Marc L.,Hoveyda, Amir H.
supporting information; experimental part, p. 9942 - 9951 (2009/02/04)
Alkylations of pyridyl-substituted ynone...
55758-02-6 Process route
-
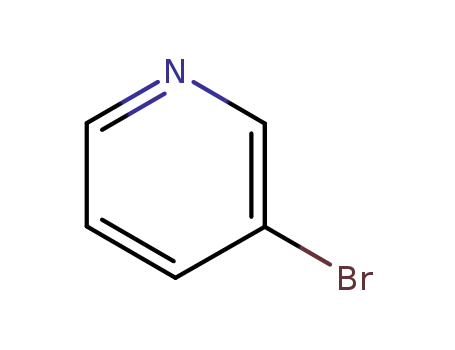
-
626-55-1,163928-56-1
3-Bromopyridine

-
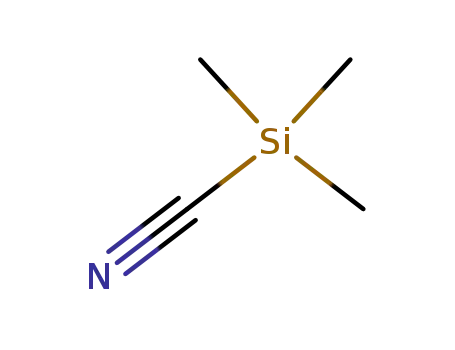
-
7677-24-9
trimethylsilyl cyanide

-
-
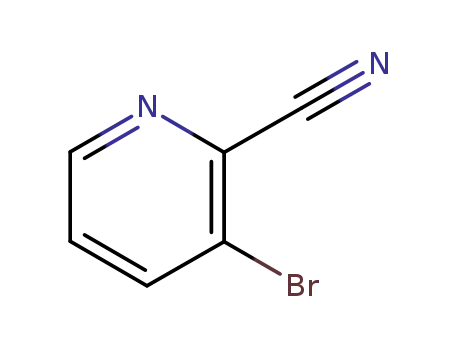
55758-02-6
3-bromopyridine-2-carbonitrile

-
-
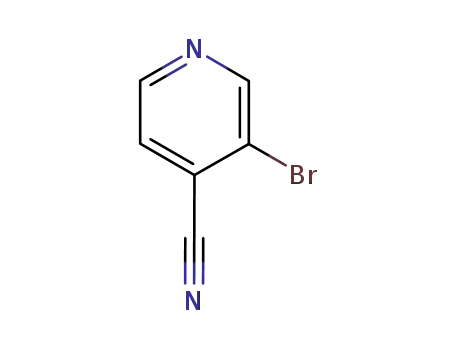
13958-98-0
3-bromo-4-cyanopyridine

-
-
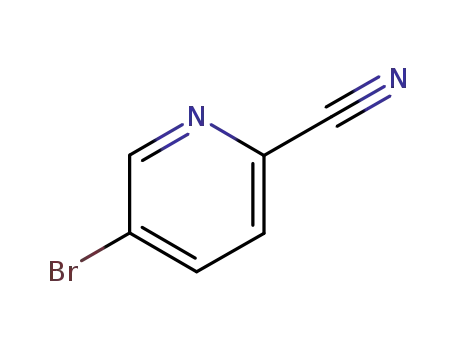
97483-77-7
2-Cyano-5-bromopyridine
| Conditions | Yield |
|---|---|
|
3-Bromopyridine;
With
trifluoromethylsulfonic anhydride;
In
chloroform;
at 20 ℃;
for 1h;
Inert atmosphere;
Sealed tube;
trimethylsilyl cyanide;
In
chloroform;
at 60 ℃;
for 3h;
Inert atmosphere;
Sealed tube;
With
4-methyl-morpholine;
In
chloroform;
at 60 ℃;
for 17h;
Overall yield = 79 %;
Inert atmosphere;
Sealed tube;
|
55% 11% 13% |
-
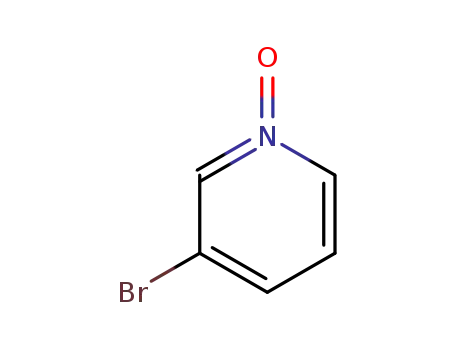
-
2402-97-3
3-bromopyridine N-oxide

-
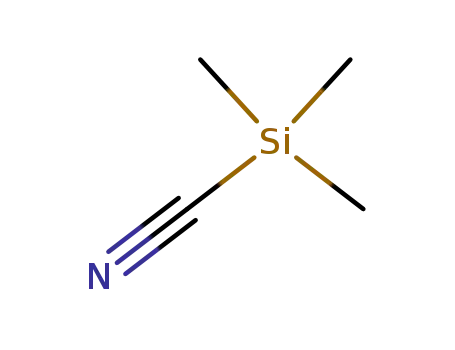
-
7677-24-9
trimethylsilyl cyanide

-

-
55758-02-6
3-bromopyridine-2-carbonitrile

-

-
97483-77-7
2-Cyano-5-bromopyridine
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
acetonitrile;
for 4h;
Heating;
|
9% 86% |
|
With
triethylamine;
In
acetonitrile;
for 4h;
Product distribution / selectivity;
Reflux;
|
|
|
With
triethylamine;
In
acetonitrile;
for 4h;
Heating / reflux;
|
55758-02-6 Upstream products
-
2402-97-3

3-bromopyridine N-oxide
-
7677-24-9
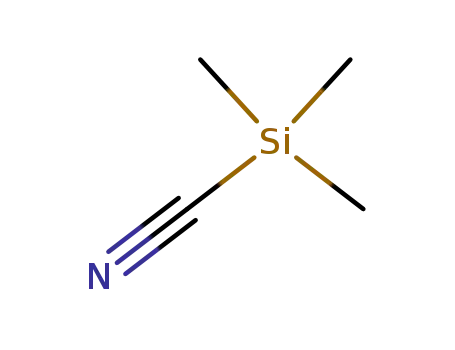
trimethylsilyl cyanide
-
626-55-1

3-Bromopyridine
55758-02-6 Downstream products
-
118160-02-4
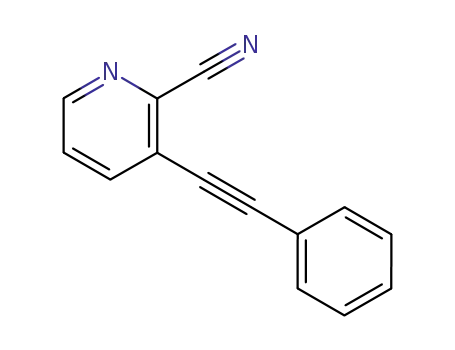
3-phenylethynylpyridine-2-carbonitrile
-
178811-40-0
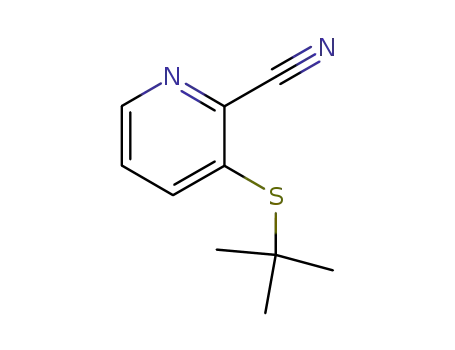
3-(tert-butylthio)picolinonitrile
-
52817-50-2
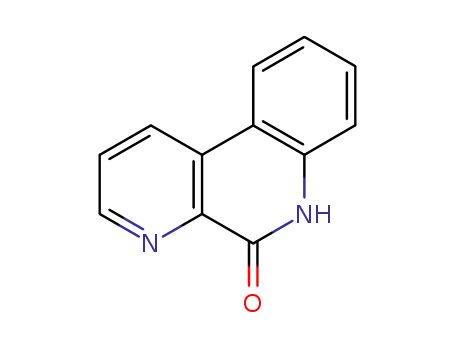
benzo[f][1,7]naphthyridin-5(6H)-one
-
53636-56-9
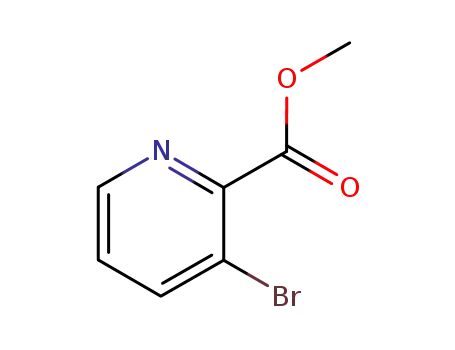
methyl 3-bromopicolinate
Relevant Products
-
3-bromo-6-methylpyridine-2-carboxylic acid
CAS:717843-48-6
-
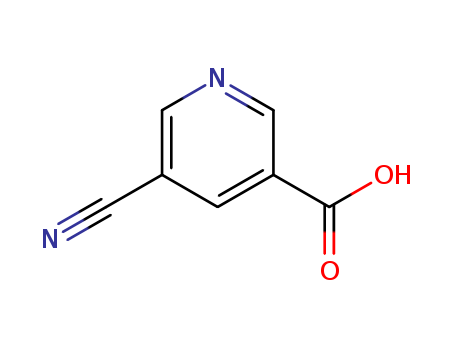
5-cyanonicotinic acid
CAS:887579-62-6
-
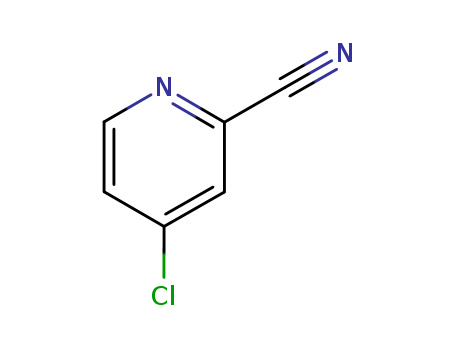
4-chlorocyanopyridine; 2-Cyano-4-chloropyridine
CAS:19235-89-3